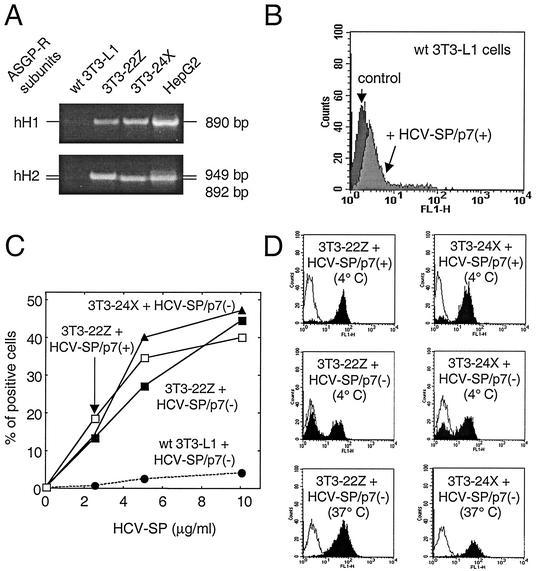
FIG. 9.
Binding of HCV-SP to ASGP-R-transfected 3T3-L1 cells. (A) 3T3-L1 cells were transfected to stably coexpress two subunits of human liver ASGP-R (hH1 and hH2). Clone 3T3-22Z coexpressed both full-length hH1 and hH2, whereas clone 3T3-24X coexpressed full-length hH1 with a truncated variant of hH2 defective for internalization but functional for binding. Total RNA was extracted from parental (wt) 3T3-L1 cells, from clones 3T3-22Z and 3T3-24X, or from HepG2 cells and subjected to reverse transcription; these cDNAs were then used to amplify by PCR a DNA fragment corresponding to either ASGP-R hH1 or hH2 subunits, as indicated. (B) Mouse fibroblasts (3T3-L1 cells) were incubated with 10 μg of HCV-SP/p7(+)/ml and subjected to the same detection protocol as in Fig. 2. (C) Both 3T3-22Z (squares) and 3T3-24X (triangles) cells, as well as parental 3T3-L1 cells (circles), were challenged with various amounts (2.5 to 10 μg/ml) of either HCV-SP/p7(+) (open symbols) or HCV-SP/p7(−) (solid symbols) and incubated for 2 h at 4°C. Cell-bound HCV-SP was detected by flow cytometry as in Fig. 2. (D) Histograms of the binding of either HCV-SP/p7(+) (top panels, 4°C) or HCV-SP/p7(−) (middle, 4°C, and bottom, 37°C, panels) to 3T3-22Z (right panels) and 3T3-24X (left panels) cells are presented.