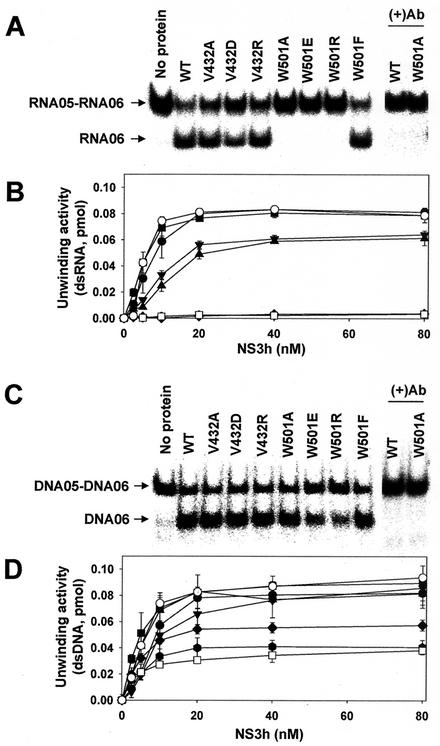
FIG. 3.
Unwinding activity of the wild-type and mutant HCV NS3 helicases on dsRNA or dsDNA substrate. (A) PAGE analysis of unwinding activity on dsRNA. A standard helicase reaction product containing 10 nM each indicated enzyme was analyzed on a native 15% polyacrylamide gel. In lanes (+)Ab, monoclonal antibody against wild-type HCV NS3 helicase was preincubated with the indicated enzyme before the enzyme reaction. (B) The dependence of the dsRNA unwinding activities on enzyme concentrations. The enzyme activity was measured for different concentration (0 to 80 nM) in the presence of dsRNA. (C) PAGE analysis of unwinding activity on dsDNA. (D) The dependence of the dsDNA unwinding activity on enzyme concentrations. The unwinding activities were quantified by determining the ratio between the amount of radioactivity associated with the release strand (RNA05 or DNA05) and the total radioactivity. The error bars represent the standard errors for two or more measurements. Symbols: •, wild type; ▪, V432A; ▴, V432D; ▾, V432R; ⧫, W501A; , W501E; □, W501R; ○, W501F.