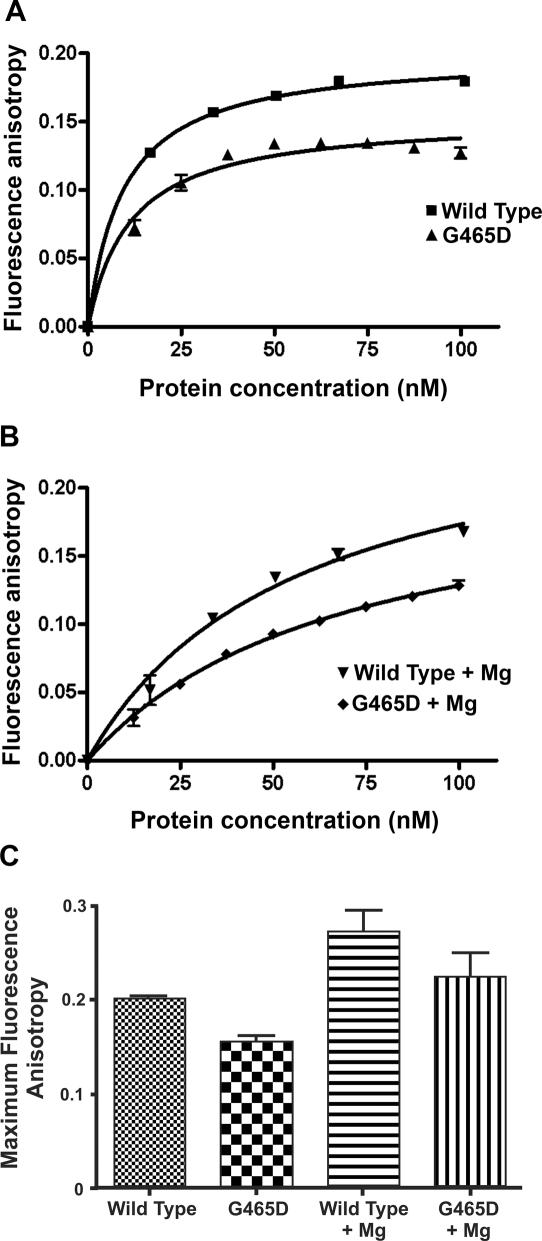
Figure 3.
Fluorescence anisotropy Bmax values for βWT and βG465D. Protein (300 nM) and HEX-labelled 40 bp oligo (1 µM) were used per experiment. MgCl2 was added to 10 mM where appropriate. Protein was added in 5 µl aliquots. For each protein concentration the average of 12 anisotropy values was taken. The average of three titrations is shown with error bars representing 1 SD from the mean. Data are presented relative to the baseline anisotropy value. (A) Anisotropy of βWT and βG465D apoproteins. (B) Anisotropy of βWT and βG465D in the presence of 10 mM MgCl2. (C) Comparison of maximum anisotropy for βWT and βG465D in the presence and absence of MgCl2.