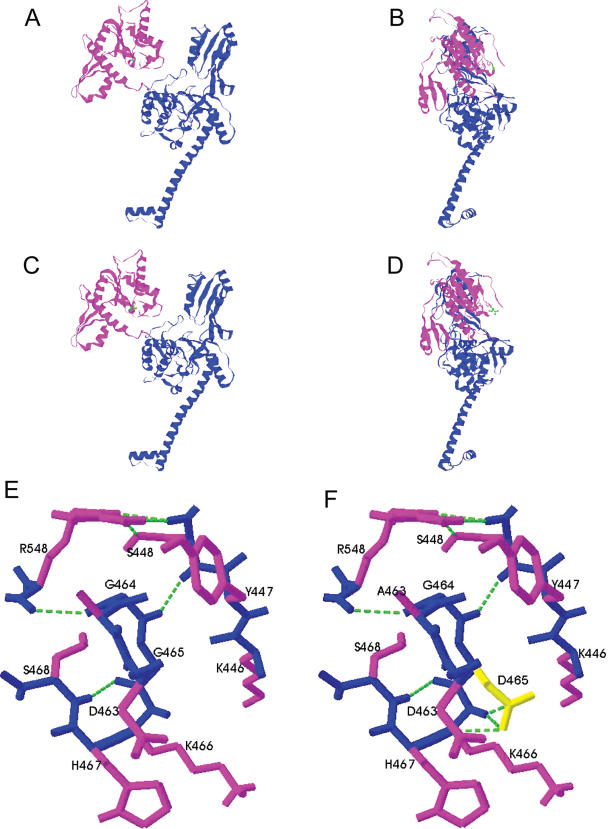
Figure 7.
Model of human topoIIβ monomer based on the yeast core structure [8]. The effect of the mutation was modelled using Swiss-PdbViewer 3.7, the figures were produced using RasMol. Shown are proteins WTβ in a front view (A) and a side view (B), and βG465D front view (C) and side view (D). The B′ domain of the protein is shown in magenta and the A′ domain in blue. Local hydrogen bonding changes close to residue 465 in the B′ domain are shown in WTβ (E) and βG465D (F). The backbone is shown in blue, side chains in magenta, residue 465 is shown in yellow and hydrogen bonds are shown as green dashed lines. The view in (E) and (F) is from the side, the same orientation as (B) and (D).