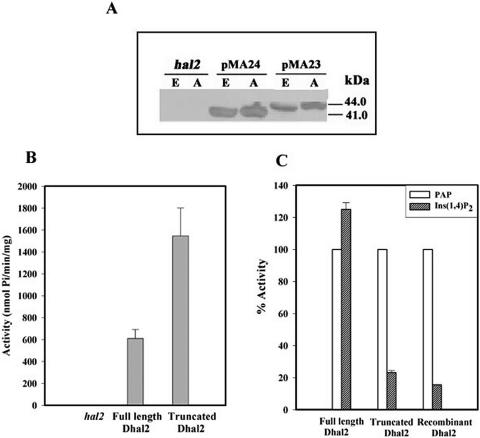
FIG. 4.
Affinity purification and enzymatic activity of ATG mutants of Dhal2p. Total protein extracts from strain RS1051 (hal2) or strain RS1051 with pMA23 (M3 ATG mutant) or pMA24 (M1/M2 ATG mutant) were subjected to affinity purification using PAP-agarose (see Materials and Methods). (A) Immunoblot of total protein extracts (E) and affinity-purified fractions (A) with anti-Dhal2p antibody The immunoblot shown is representative of three different experiments. (B) An equal amount of protein (0.2 μg) from each fraction was incubated with PAP, and the amount of Pi released was measured as described in Materials and Methods. The PAP nucleotidase activity of each fraction is expressed as nmol of Pi/min/mg. (C) Comparison of activity of each fraction against PAP and inositol-1,4-bisphosphate. The activity of each form against inositol-1,4-bisphosphate is expressed as a percentage of the activity of the particular form against PAP. As a control, the activity of the recombinant truncated Dhal2p was measured. Results of three independent experiments are presented and error bars represent means ± standard deviations.