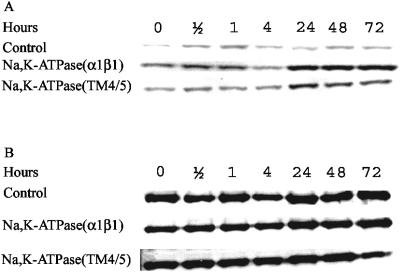
FIG. 6.
Time-dependent phosphorylation of eIF-2α by Gcn2p kinase during heterologous expression of α1β1 Na,K-ATPase or empty vector. Yeast cells were grown as described in Materials and Methods and harvested 0, 4, 24, 48, and 72 h after galactose induction. Cytosolic fractions were isolated, and equal amounts of total cytoplasmic protein (40 μg) were analyzed by Western blotting using a polyclonal antibody that specifically recognizes eIF-2α phosphorylated at serine 51 (A) or a polyclonal antibody that recognizes phosphorylated and nonphosphorylated forms of eIF-2α (Β).