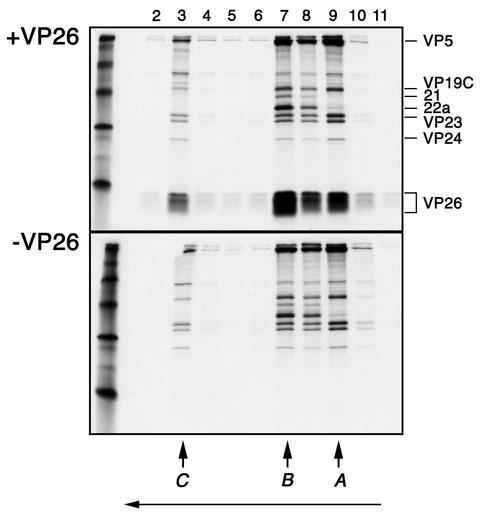
FIG. 1.
In vitro capsid binding assay to study interaction of VP26 with the capsid. Capsids that lack VP26 were isolated from sucrose gradients following sedimentation of [35S]methionine-radiolabeled lysates prepared from KΔ26Z-infected cells. The UL35 ORF in pGEM3Z was used as a template to synthesize [35S]methionine-labeled VP26. Capsids and in vitro-translated protein (+VP26) were incubated at RT for 90 min (continuous rotation) and then sedimented through 20 to 50% sucrose gradients (top panel). In parallel experiments, similarly prepared capsids were incubated with reticulolysate (−VP26) and analyzed as described above (lower panel). The fractions (2 to 11) collected following sedimentation were analyzed by SDS-PAGE (17% acrylamide). The gels were processed as described in Materials and Methods and dried prior to autoradiography, and the autoradiograph obtained is shown in the figure. The direction of sedimentation was from right to left (indicated by the arrow at the bottom of the figure). The positions at which C, B, and A capsids sediment are indicated at the bottom of the panel. The positions of the capsid proteins in the gel are indicated to the right of the top panel. Radioactivity corresponding to in vitro-synthesized VP26 was detected in the fractions containing capsid proteins, indicating VP26 binding to capsids. Protein standards were loaded in the extreme left lanes and correspond to 220, 97, 66, 45, 30, and 14.3 kDa.