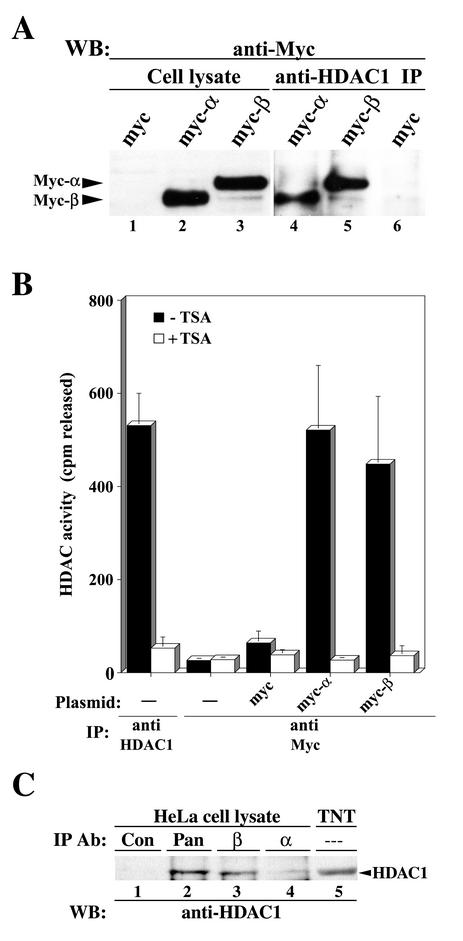
FIG. 3.
A functional HDAC1 coimmunoprecipitates with hMI-ER1α and hMI-ER1β in vivo. (A) Cell lysates from HeLa cells transiently transfected with myc tag empty vector or myc-α or myc-β plasmid were prepared, and equivalent amounts of protein from each sample were either added directly to sample buffer (lanes 1 to 3) or subjected to immunoprecipitation (IP) with anti-HDAC1 (lanes 4 to 6); Western blot (WB) analysis was performed using anti-Myc. The positions of the Myc-α and Myc-β proteins are indicated. (B) HeLa cells (1.5 × 105 cells per sample) were transfected with myc tag empty vector or myc-α or myc-β plasmid and lysed, and the supernatants were subjected to IP with anti-Myc. Additional controls consisted of mock-transfected HeLa cell extracts immunoprecipitated with anti-HDAC1 or anti-Myc. Immunoprecipitates were assayed for HDAC activity in the presence or absence of 300 nM TSA as described in Materials and Methods. Shown are the average values and standard deviations from three independent experiments. (C) HeLa cell lysates were subjected to IP with nonimmune serum (lane 1) or anti-pan hMI-ER1 (lane 2), anti-hMI-ER1β-specific (lane 3), or anti-hMI-ER1α-specific (lane 4) antiserum. HDAC1 protein from an in vitro TNT mixture was loaded in lane 5. Western blot (WB) analysis was performed using anti-HDAC1. The position of the HDAC1 protein is indicated.