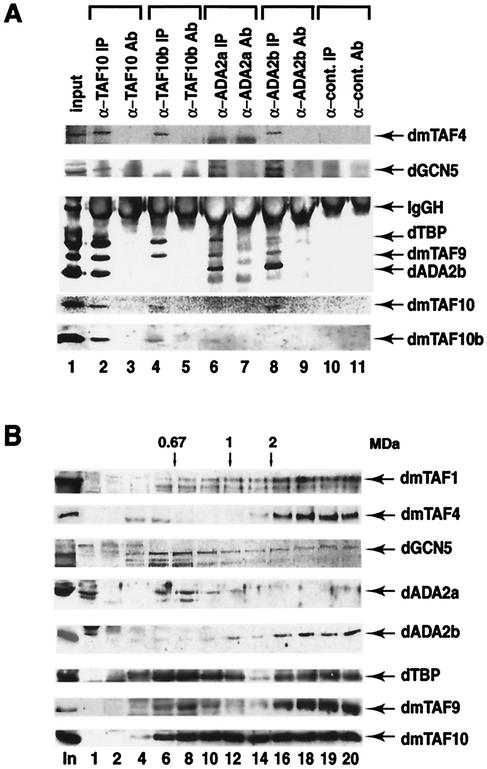
FIG. 4.
dADA2a and dADA2b are subunits of different GCN5 HAT-containing multiprotein complexes. dADA2a and dADA2b coimmunoprecipitate with dGCN5 and with several different dTAFs. Proteins from nuclear extracts were immunoprecipitated with affinity-purified anti-dTAF10, anti-dTA10b, anti-dADA2a, and anti-dADA2b PAbs and a nonrelated PAb raised against a human protein, as indicated. The input nuclear fraction (input), the immunoprecipitated protein A-Sepharose antibody-bound proteins (IP), and the protein A-Sepharose bound PAbs alone (Ab) were resolved by SDS-PAGE (10 or 15% polyacrylamide) and transferred. The blots were then probed with antibodies raised against the indicated proteins. (B) Glycerol gradient sedimentation analysis of Drosophila embryo nuclear extract. A 200-μl volume of TRAX was loaded onto a 10 to 30% glycerol gradient and centrifuged for 4 h at 50 krpm, using an SW60 Ti rotor. The sedimentation standards, thyroglobulin (669 kDa), ferritin (440 kDa), catalase (232 kDa), lactate dehydrogenase (140 kDa), and albumin (67 kDa), were centrifuged in parallel gradients. Fractions of 200 μl were collected. Aliquots (20 μl) from the indicated fractions were resolved in parallel with the unfractioned nuclear extract (In) by SDS-PAGE (10% polyacrylamide), transferred to a filter, and probed with the indicated PAbs. The position of the 0.67-MDa molecular mass marker and some extrapolated molecular masses are indicated at the top. The numbers of the analyzed fractions are indicated at the bottom. Note that only the 42-kDa form of dADA2b is shown; however, the 70-kDa form showed exactly the same migration pattern.