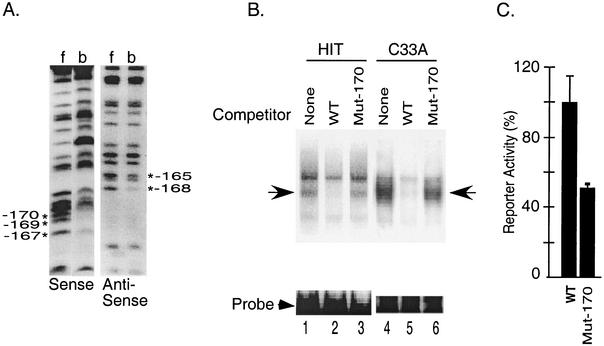
FIG. 3.
Characterization of the factors binding to the upstream element in the secretin enhancer. (A) Partially methylated probes were incubated with HIT cell nuclear extract in an electrophoretic mobility shift assay. Following electrophoresis, free probe (f) and probe bound to proteins in the upstream element complex (b) were eluted from the gel, cleaved with piperidine, and resolved on an 8% sequencing gel to generate G ladders. *, G residues that are important for complex formation. (B) The upstream element probe was incubated in a gel shift reaction with the nuclear extract from HIT cells or C33A cells in the absence (lane 1) or presence of a 100-fold molar excess of the wild type (WT) (lane 2) or the Mut-170 (lane 3) competitor. Arrow, specific DNA-protein complex. (C) HIT cells were transiently transfected with a secretin-luciferase reporter plasmid (WT) or the same reporter with a point mutation at −170 (Mut-170). Luciferase activity was measured in cell extracts 20 h later. Results are expressed as percentages relative to the wild-type reporter and are the means ± SEM normalized for transfection efficiency for at least three independent experiments.