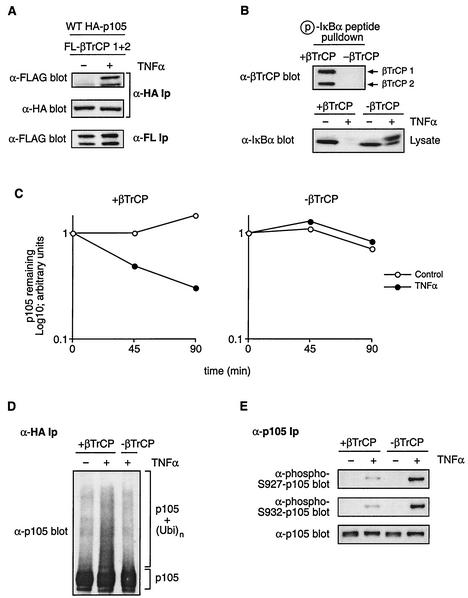
FIG. 6.
βTrCP is required for TNF-α-induced p105 ubiquitination and proteolysis. (A) HeLa cells stably transfected with HA-p105 were transiently transfected with vectors encoding FL-βTrCP1 and FL-βTrCP2. After 48-h culture, cells were stimulated for 15 min with TNF-α or left unstimulated. Anti-HA immunoprecipitates (Ip) were Western blotted with the indicated antibodies. FL-βTrCP expression in cell lysates was confirmed by anti-FL MAb immunoprecipitation and Western blotting. WT, wild type. (B) HeLa cells were pretreated with βTrCP siRNAs complementary to both βTrCP1 and βTrCP2 (−βTrCP) or with control buffer (+βTrCP). After 72 h, cells were stimulated with TNF-α (15 min) or left unstimulated and then lysed. In the upper gel, βTrCP expression of unstimulated cells was determined by Western blotting of phospho-IκBα peptide pulldowns. In the lower gel, cell lysates were Western blotted for IκBα. (C) HeLa cells stably expressing HA-p105 were pretreated as described for panel B. Turnover of HA-p105 in TNF-α-stimulated and control unstimulated cells was determined by pulse-chase metabolic labeling as done for that shown in Fig. 2. Similar results were obtained on two other occasions. (D) βTrCP expression in HA-p105 HeLa cells was suppressed by siRNA pretreatment as described for panel B. HA-p105 ubiquitination induced by 15-min TNF-α stimulation was determined as described in the legend to Fig. 5. (E) βTrCP expression was decreased by siRNA treatment as described for panel B. Phosphorylation of endogenous p105 after TNF-α treatment (15 min) was determined by immunoprecipitation and Western blotting with the indicated antibodies.