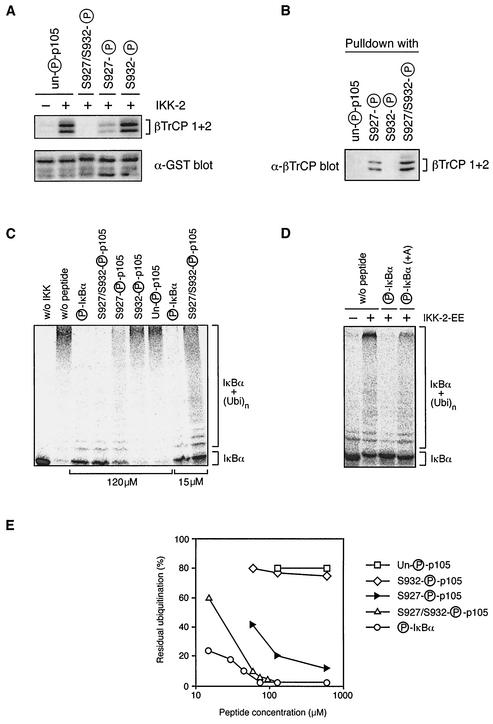
FIG. 7.
Efficient binding of βTrCP to p105 requires phosphorylation of p105 serines 927 and 932. (A) GST-p105758-967 fusion protein, phosphorylated by His6-IKK2 or left unphosphorylated, was coupled to glutathione-Sepharose beads and used to affinity purify βTrCP translated and labeled with [35S]methionine in vitro. The indicated peptides were added to a final concentration of 100 μg/ml during the pulldown. Isolated βTrCP was detected by autoradiography of SDS-8% PAGE gels. (B) The indicated peptides were coupled to Affi-Gel 10 beads and used as affinity matrices to isolate from HeLa cell lysates endogenous βTrCP, which was detected by Western blotting. (C and D) IκBα, translated and labeled in vitro with [35S]methionine, was first phosphorylated with recombinant IKK2-EE. Labeled protein was then ubiquitinated in vitro (26) in the presence of the indicated peptides or with no added peptide (w/o peptide). Ubiquitination of unphosphorylated IκBα (w/o IKK) is shown as a negative control. IκBα ubiquitination was revealed by autoradiography of SDS-8% PAGE gels. (E) The extent of IκBα ubiquitination in vitro (carried out as described for panel C) was quantified on a phosphorimager over a range of competing peptide concentrations.