FIG. 4.
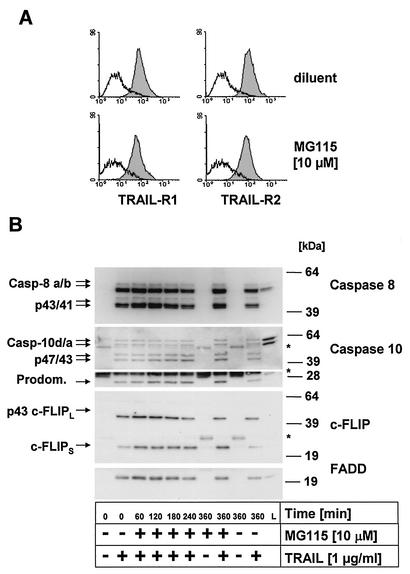
Caspases 8 and 10 are recruited and cleaved at the native TRAIL DISC independent of MG115. (A) Primary keratinocytes were treated with 10 μM MG115 or diluent for 6 h and subsequently analyzed for surface expression of TRAIL-R1 and TRAIL-R2 by fluorescence-activated cell sorting. MG115 does not modulate TRAIL-R1 or TRAIL-R2 surface expression on primary keratinocytes. (B) Analysis of the native TRAIL DISC in primary keratinocytes. Cells were treated for the indicated times with MG115 or diluent alone. The stimulated TRAIL DISC (+) or unstimulated receptors (−) were precipitated with Flag-TRAIL precomplexed with 2 μg of anti-Flag antibodies per ml (clone M2). The resulting protein complexes were separated by SDS-PAGE and analyzed by Western blot for components of the TRAIL DISC. The adaptor protein FADD was detectable only when stimulated receptors were precipitated. Also, recruitment of the two isoforms of caspase 8, caspase 8a and 8b, was detected only following stimulation of TRAIL receptors. In addition, the intermediate cleavage products, p43/p41, of the two isoforms of caspase 8 were detectable in the DISC. For caspase 10, the full-length forms caspase 10d (p59) and caspase 10a (p55) as well as the cleavage products p47/43 and the p25 fragment, corresponding to the prodomain (Prodom.), were detectable in the immunoprecipitates. Analysis of cFLIP levels revealed that both isoforms, cFLIPL and cFLIPS, were present in the TRAIL DISC, with comparable levels in control cells and MG115-treated cells. For cFLIPL, only the p43 cleavage product was detectable in the DISC, indicative of proteolytic processing by active caspases in the DISC. The migration position of the IgG heavy and light chains of the precipitating anti-Flag M2 antibody as well as that of protein G cross-reacting with the antibody used for Western blot detection (caspase 10, lower part) are indicated by asterisks.