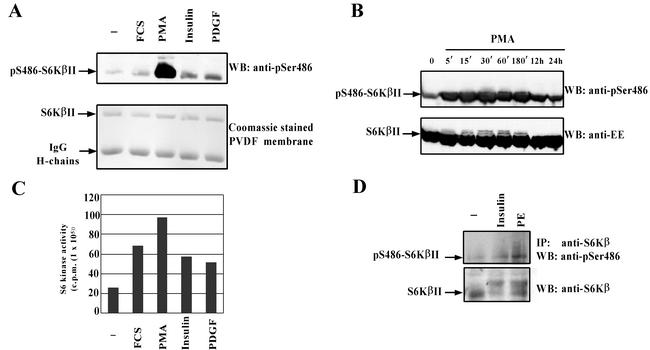
FIG. 4.
S6KβII is phosphorylated at Ser486 in response to different mitogenic stimuli. HEK 293 cells were transiently transfected with wild-type EE-S6KβII, serum starved, and stimulated with 10% FCS, 1 μM PMA, 100 nM insulin, 50 ng of PDGF/ml, or vehicle alone (−). Recombinant S6KβII was immunoprecipitated with anti-EE antibody and used for the in vitro S6K assay (B) or analyzed by Western blotting (WB) with anti-pS486 antibody (A). (C) Time course phosphorylation of S6KβII at Ser486 in PMA-treated HEK 293 cells. HEK 293 cells were transiently transfected with wild-type EE-S6KβII, serum starved for 24 h, and stimulated with 1 μM PMA for the indicated period of time. Cell lysates were analyzed by Western blotting with anti-pS486 or anti-EE antibodies. (D) Phosphorylation of endogenous S6Kβ at Ser486 in phenylephrine (PE)-stimulated cardiomyocytes. Isolated cardiomyocytes were treated with 10 μM phenylephrine, 10 nM insulin, or vehicle alone for 30 min. Native S6Kβ was immunoprecipitated (IP) from lysed cells with anti-C-terminal antibodies. Immune complexes were separated by SDS-PAGE and immunoblotted with anti-pS486 antibody. The results presented have been reproduced in three independent experiments. IgG, immunoglobulin G.