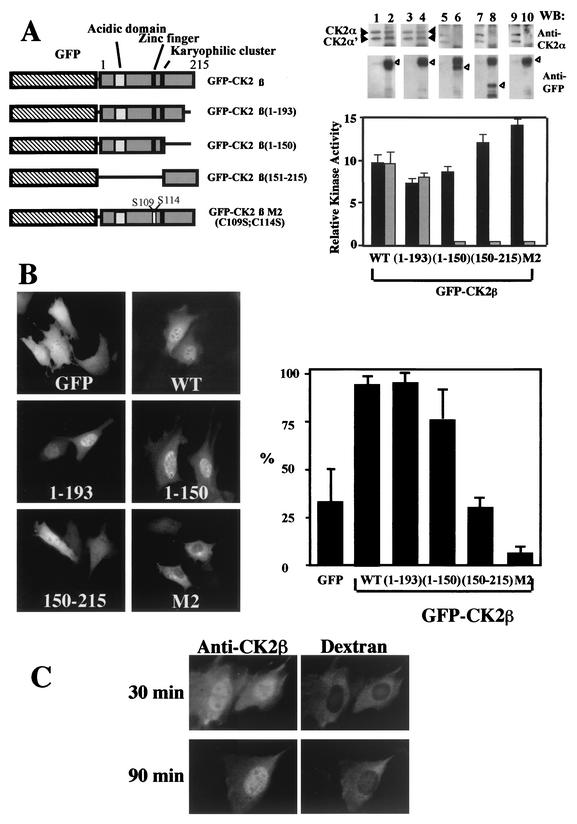
FIG. 2.
The C-terminal domain is not required for nuclear import of CK2β. (A) NIH 3T3 cells were transiently transfected with different GFP-CK2β constructs as illustrated in the diagram. Extracts from NIH 3T3 cells transfected with the different constructs were immunoprecipitated with anti-CK2β (black bars) or anti-GFP (grey bars) antibodies, and the corresponding immunoprecipitates were used for CK2 activity determination. Anti-CK2β immunoprecipitates (lanes 1, 3, 5, 7, and 9) or anti-GFP immunoprecipitates (lanes 2, 4, 6, 8, and 10) were also analyzed by Western blotting (WB) for the presence of CK2 subunits using anti-CK2α (solid arrowheads) or anti-GFP (open arrowheads) antibodies. WT, wild type; error bars, standard deviations. (B) Transfected cells were observed for protein localization. Bar, 10 μm. Cells were also scored according to their nuclear staining expressed as a percentage of transfected cells (error bars, standard deviations). (C) Dynamics of CK2β nuclear import. CK2β expressed in E. coli was microinjected into the cytoplasm of NIH 3T3 cells in interphase with dextran Texas Red to identify injected cells. At the indicated time points after injection, cells were fixed and CK2β was detected by indirect immunofluorescence using anti-CK2β antibodies.