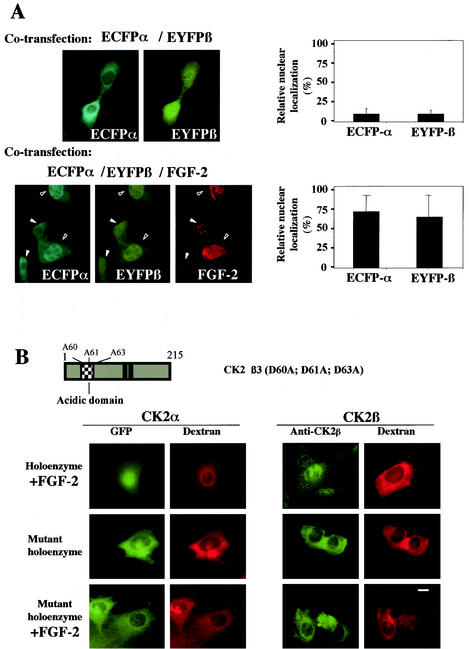
FIG. 5.
FGF-2 triggers the nuclear import of CK2 holoenzyme in living cells. (A) NIH 3T3 cells were cotransfected with the following plasmids: ECFP-CK2α/EYFP-CK2β in the absence or presence of an FGF-2 construct. Sixteen hours after transfection a dual-color imaging analysis was performed and the expression of FGF-2 was detected by indirect immunofluorescence using an anti-FGF-2 antibody. Note that in low-FGF-2-expressing cells (solid arrowheads), both CK2 subunits were mainly cytoplasmic, whereas in high-FGF-2-expressing cells (open arrowheads), they were nuclear. Cotransfected cells (150 to 300) were also scored according to whether the level of fluorescence of GFP was higher in the nucleus than in the cytoplasm, and the results were expressed as a percentage of the total number of transfected cells. Error bars, standard deviations. (B) A GFP-CK2α/CK2β complex was incubated with FGF-2 in vitro before injection into the cytoplasm of NIH 3T3 cells in interphase along with 70-kDa dextran Texas Red. Localization of the GFP CK2α was followed by monitoring GFP fluorescence every 15 s. Images shown are after 10 min. Localization of CK2β was detected by indirect immunofluorescence using anti-CK2β antibodies on cells fixed 10 min after injection. Microinjection of a complex between GFP CK2α and the CK2β3 mutant was performed as above in the absence or presence of FGF-2. Representative cells of at least two different experiments are shown for all panels. Bar, 10 μm. A schematic diagram of CK2β3 mutant is shown.