FIG. 7.
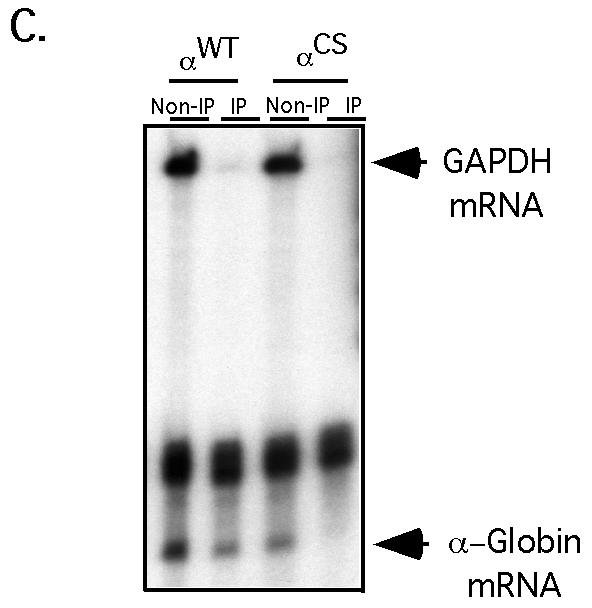
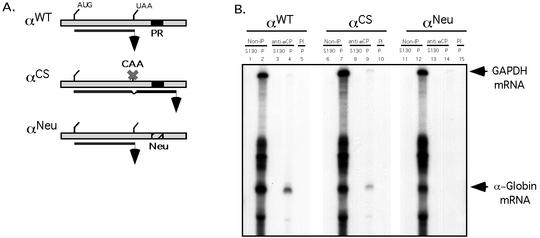
Interaction of αCP2 with α-globin mRNA is uniquely dependent on the 3′ UTR poly(C)-rich region and is sensitive to displacement by an antiterminated 80S. (A) Expression of hα-globin mRNAs with distinct 3′ UTRs (WT, Neu, and CS) in MEL/tTA cells. The structures of the three encoded mRNAs under the control of a tet promoter are shown. The positions of the translation start site (AUG), termination site (UAA), αCP binding site (protected region [PR]), and the antitermination mutation in the αCS (UAA → CAA) are shown. The cross-hatched box represents the substitution of a neutral sequence for the PR motif. (B) αCP binding on hα-globin mRNA is restricted to the C-rich 3′ UTR stability motif. Each of the indicated α-globin mRNAs was expressed in transfected cells from a corresponding plasmid. After 24 h of induction of expression in TET-deficient medium, the cells were lysed and the clarified cytoplasmic (S20) extracts were layered onto a 30% sucrose cushion. The isolated prepolysomal (S130) and polysomal (P) fractions were separately immunoprecipitated with antibody to αCP2 and αCP2-KL or with preimmune serum (PI). RNA was extracted from the starting material and from each immunoprecipitate. hα-globin and GAPDH mRNAs were detected by RPA. The origin of each sample is indicated above its respective lane. The positions of the RPA probes are indicated to the right of the gel. (C) Selective dissociation of αCP from the antiterminated αCS mRNA. MEL/tTA cells were separately transfected with pTet-WT and pTet-CS plasmids. The transfected genes were transcriptionally induced for 24 h in TET-deficient medium. TET was then added back to the medium at a concentration of 500 ng/ml for an additional 2 h. The cells were subsequently lysed and clarified (S10), and RNP complexes were immunoprecipitated with anti-αCP2 sera. mRNA content in the precipitate was analyzed by RPA as described for panel B. These studies were carried out independently three times with consistent results.