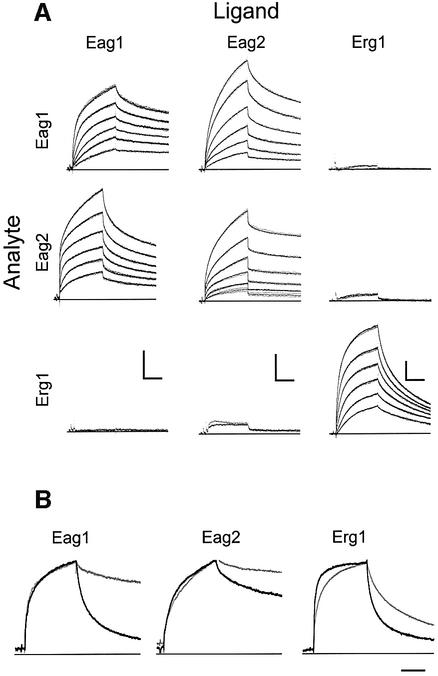
Fig. 4. (A) Kinetics of TCC peptide homo- and heteromeric assembly. Peptides were immobilized on the sensor surface (ligand, columns) and homomeric and heteromeric interactions were studied by surface plasmon resonance. Peptides in solution (analyte, rows) were brought into contact with the ligands for 480 s (association) and dissociation was monitored for 600 s (dots) at 1 Hz. When the injection of peptide at 3 µM yielded more than 5 RU, the interaction was studied in triplicate at decreasing concentrations of 3 µM, 1 µM, 300 nM, 100 nM, 30 nM and 10 nM, leading to decreasing responses. Fits are superimposed as solid lines. Scale bars: 200 s; 10 RU. (B) Kinetics of the heteromeric assembly of TCCEag1L20Y, TCCEag2L13Y and TCCErg1L20Y (as analytes) with the corresponding wild-type peptides (grey, as ligands). The corresponding homomeric interaction between wild-type peptides is shown in black. Scale bar: 200 s.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.