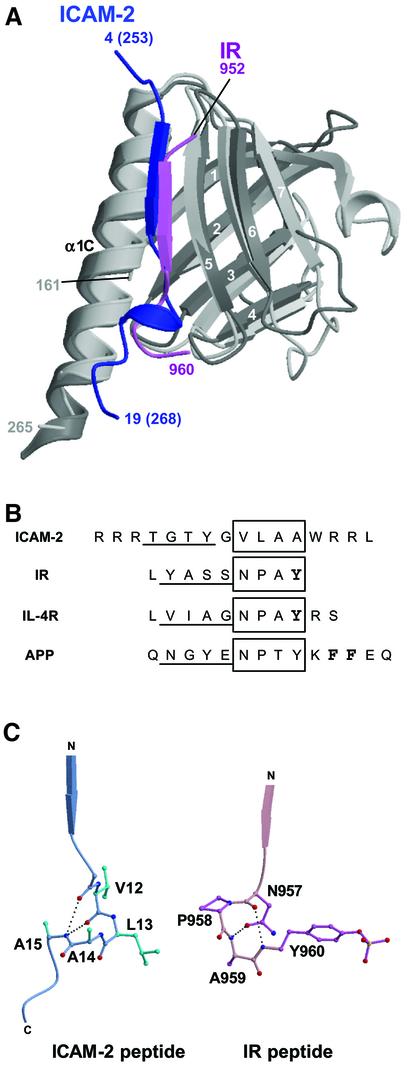
Fig. 5. Comparison of the FERM–ICAM-2 complex with the related PTB–peptide complexes. (A) Superposition of the radixin FERM subdomain C (gray) bound to the ICAM-2 tail (blue) on the IRS-1 PTB domain (light gray) bound to the IR peptide (pink). (B) Peptide alignment based on the superimposed complex structures. The β-strands formed in the complex formations are underlined. The 310 helix in ICAM-2 and the NPxY motif sequences in the PTB domain-binding (IR, IL-4R and APP) peptides are boxed. The phosphotyrosine residues are in bold in the IR and IL-4R peptides. In the APP peptide, two Phe residues anchoring the peptide to the X11 PTB domain are also in bold. (C) Comparison of the 310 helix in the ICAM-2 peptide bound to the radixin FERM domain with the β-turn of the NPxY motif in the IR peptide bound to the IRS-1 PTB domain. The intra-peptide hydrogen bonds are shown as dotted lines.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.