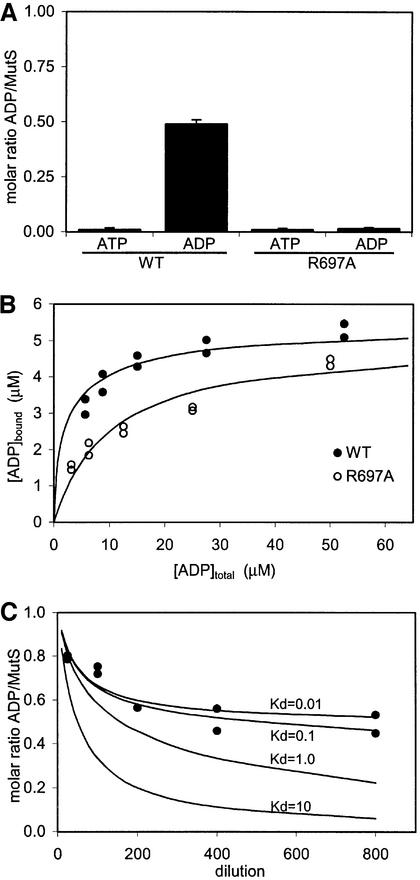
Fig. 1. The MutS dimer has two non-equivalent ADP-binding sites. (A) Amount of ATP and ADP retained by purified MutS (wild-type and R697A) as measured in a luciferase assay. (B) Filter binding studies on wild-type and R697A MutS (5 µM) with increasing amounts of radiolabelled ADP. (C) Diluting out ADP from wild-type MutS. Black dots show the amount of radiolabelled ADP retained by MutS after dilution in a filter binding assay. Lines indicate theoretical dilution curves of a two-site binding model, with one site having a Kd of 10 µM and the second site as indicated in the graph.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.