Abstract
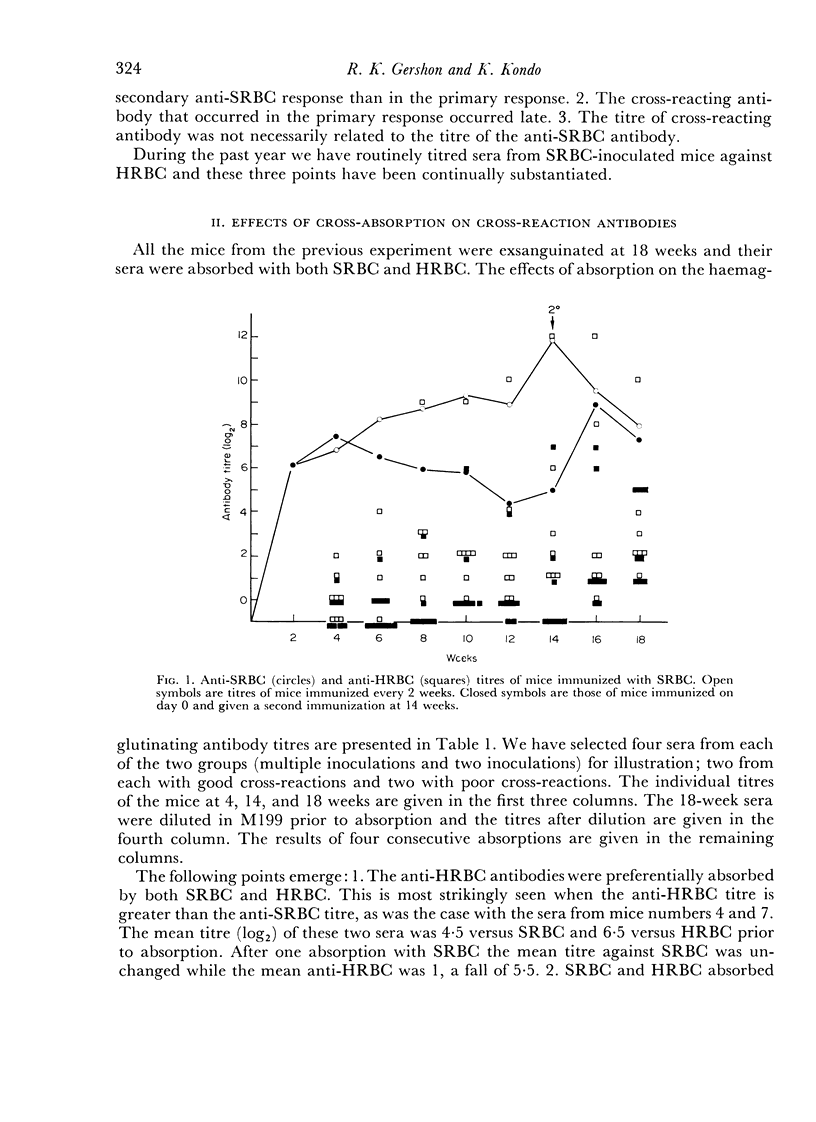
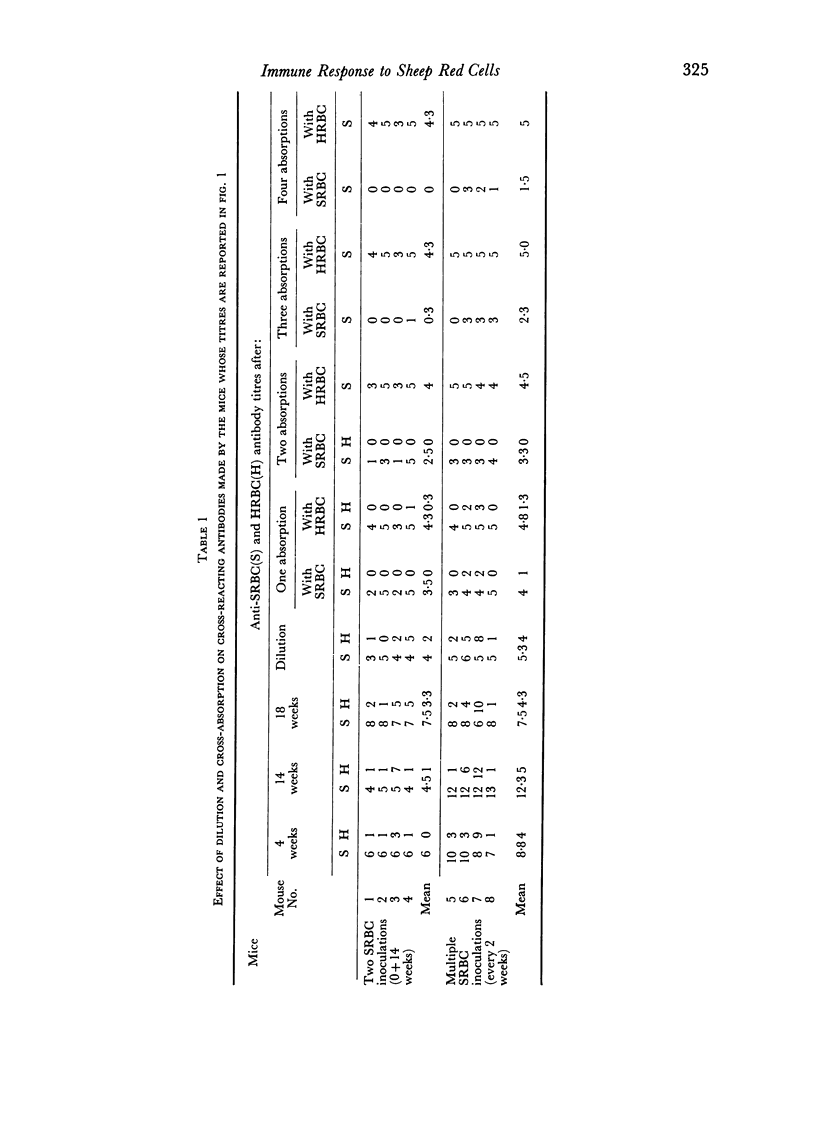
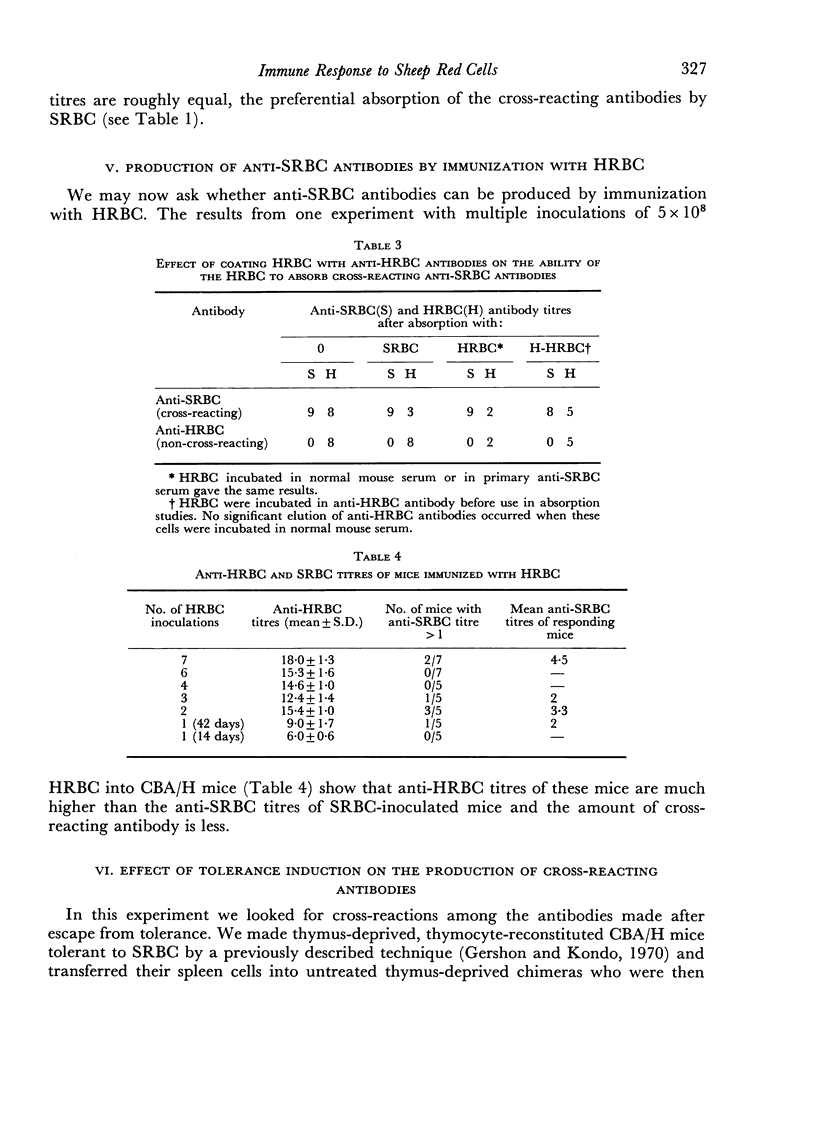
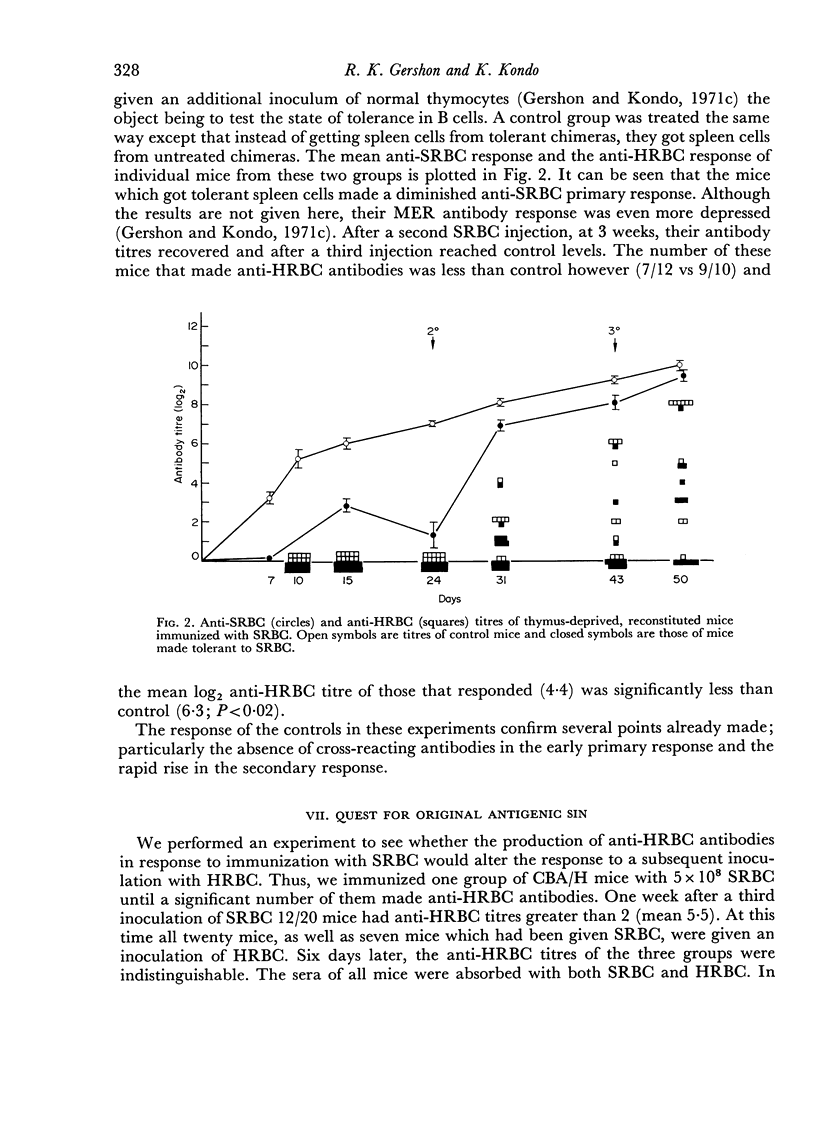
We found that some mice immunized with sheep red blood cells (SRBC) made antibodies that agglutinated horse RBC (HRBC). Absorption studies indicated that the cross-reacting antibodies were highly avid. They appeared either late in a primary response, in secondary responses, or after multiple immunizations. Thus, the antibody response to SRBC loses specificity with time and is a further example of the degeneracy of the immune response. The similarities between the system we described and previously studied examples of degeneracy suggest that their bases may be the same; reactions of high affinity antibodies with related determinants. This interpretation was strengthened by the observation that antibodies made by mice which had recently escaped from tolerance were particularly deficient in cross-reacting subsets. The possible pitfalls of using hyperimmune serum in tests requiring high degrees of immunological specificity were emphasized.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADLER F. L. STUDIES ON MOUSE ANTIBODIES. I. THE RESPONSE TO SHEEP RED CELLS. J Immunol. 1965 Jul;95:26–38. [PubMed] [Google Scholar]
- Albright J. F., Omer T. F., Deitchman J. W. Antigen competition: antigens compete for a cell occurring with limited frequency. Science. 1970 Jan 9;167(3915):196–198. doi: 10.1126/science.167.3915.196. [DOI] [PubMed] [Google Scholar]
- Baker P. H., Stashak P. W. Quantitative and qualitative studies on the primary antibody response to pneumococcal polysaccharides at ehe cellular level. J Immunol. 1969 Dec;103(6):1342–1348. [PubMed] [Google Scholar]
- Bast R. C., Jr, Manseau E. J., Dvorak H. F. Heterogeneity of the cellular immune response. I. Kinetics of lymphocyte stimulation during sensitization and recovery from tolerance. J Exp Med. 1971 Feb 1;133(2):187–201. doi: 10.1084/jem.133.2.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown P. C., Glynn L. E. The recovery of immunological reactivity to some synthetic polypeptides containing proline after the induction of specific immunological tolerance. Immunology. 1969 Dec;17(6):943–953. [PMC free article] [PubMed] [Google Scholar]
- DRESSER D. W. Specific inhibition of antibody production. II. Paralysis induced in adult mice by small quantities of protein antigen. Immunology. 1962 May;5:378–388. [PMC free article] [PubMed] [Google Scholar]
- Davies A. J. The thymus and the cellular basis of immunity. Transplant Rev. 1969;1:43–91. doi: 10.1111/j.1600-065x.1969.tb00136.x. [DOI] [PubMed] [Google Scholar]
- Dietrich F. M., Dukor P. The immune response to heterologous red cells in mice. II. Antibody formation to red cells from species taxonomically related to sheep or mouse. Immunology. 1967 Dec;13(6):585–595. [PMC free article] [PubMed] [Google Scholar]
- EISEN H. N., SISKIND G. W. VARIATIONS IN AFFINITIES OF ANTIBODIES DURING THE IMMUNE RESPONSE. Biochemistry. 1964 Jul;3:996–1008. doi: 10.1021/bi00895a027. [DOI] [PubMed] [Google Scholar]
- Eidinger D., Pross H. F., Kerbel R. S., Baines M. G., Ackerman A., Khan S. A. Further studies of competition of antigens. I. Variation in immunosuppression induced by alterations of dosage, route of injection, nature of antigen, and immunological status of host. Can J Microbiol. 1971 Jun;17(6):803–812. doi: 10.1139/m71-127. [DOI] [PubMed] [Google Scholar]
- Fazekas de St Groth, Webster R. G. Disquisitions of Original Antigenic Sin. I. Evidence in man. J Exp Med. 1966 Sep 1;124(3):331–345. doi: 10.1084/jem.124.3.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershon R. K., Kondo K. Antigenic competition between heterologous erythrocytes. I. Thymic dependency. J Immunol. 1971 Jun;106(6):1524–1531. [PubMed] [Google Scholar]
- Gershon R. K., Kondo K. Antigenic competition between heterologous erythrocytes. II. Effect of passive antibody administration. J Immunol. 1971 Jun;106(6):1532–1539. [PubMed] [Google Scholar]
- Gershon R. K., Kondo K. Cell interactions in the induction of tolerance: the role of thymic lymphocytes. Immunology. 1970 May;18(5):723–737. [PMC free article] [PubMed] [Google Scholar]
- Gershon R. K., Kondo K. Degeneracy of the immune response to sheep red cells. Thymic dependency. Immunology. 1972 Sep;23(3):335–342. [PMC free article] [PubMed] [Google Scholar]
- Gershon R. K., Kondo K. Infectious immunological tolerance. Immunology. 1971 Dec;21(6):903–914. [PMC free article] [PubMed] [Google Scholar]
- Gershon R. K., Paul W. E. Effect of thymus-derived lymphocytes on amount and affinity of anti-hapten antibody. J Immunol. 1971 Mar;106(3):872–874. [PubMed] [Google Scholar]
- Gilden R. V., Parks W. P., Huebner R. J., Todaro G. J. Murine leukaemia virus group-specific antigen in the C-type virus-containing human cell line, ESP-1. Nature. 1971 Sep 10;233(5315):102–103. doi: 10.1038/233102a0. [DOI] [PubMed] [Google Scholar]
- Haskill J. S. Density distribution analysis of antigen-sensitive cells in the rat. J Exp Med. 1969 Oct 1;130(4):877–893. doi: 10.1084/jem.130.4.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little J. R., Border W., Freidin R. The binding reactions of antibodies specific for the 2,6-dinitrophenyl group. J Immunol. 1969 Oct;103(4):809–817. [PubMed] [Google Scholar]
- Little J. R., Eisen H. N. Specificity of the immune response to the 2,4-dinitrophenyl and 2,4,6-trinitrophenyl groups. Ligand binding and fluorescence properties of cross-reacting antibodies. J Exp Med. 1969 Feb 1;129(2):247–265. doi: 10.1084/jem.129.2.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MILLER J. F. Studies on mouse leukaemia. The role of the thymus in leukaemogenesis by cell-free leukaemic filtrates. Br J Cancer. 1960 Mar;14:93–98. doi: 10.1038/bjc.1960.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J. F., Mitchell G. F. Cell to cell interaction in the immune response. I. Hemolysin-forming cells in neonatally thymectomized mice reconstituted with thymus or thoracic duct lymphocytes. J Exp Med. 1968 Oct 1;128(4):801–820. doi: 10.1084/jem.128.4.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J. F., Mitchell G. F. Cell to cell interaction in the immune response. V. Target cells for tolerance induction. J Exp Med. 1970 Apr 1;131(4):675–699. doi: 10.1084/jem.131.4.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell G. F., Miller J. F. Immunological activity of thymus and thoracic-duct lymphocytes. Proc Natl Acad Sci U S A. 1968 Jan;59(1):296–303. doi: 10.1073/pnas.59.1.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchison N. A. The carrier effect in the secondary response to hapten-protein conjugates. I. Measurement of the effect with transferred cells and objections to the local environment hypothesis. Eur J Immunol. 1971 Jan;1(1):10–17. doi: 10.1002/eji.1830010103. [DOI] [PubMed] [Google Scholar]
- Möller G., Sjöberg O. Effect of antigenic competition on antigen-sensitive cells and on adoptively transferred immunocompetent cells. Cell Immunol. 1970 May;1(1):110–121. doi: 10.1016/0008-8749(70)90064-x. [DOI] [PubMed] [Google Scholar]
- Nussenzweig V., Benacerraf B. Antihapten antibody specificity and L chain type. J Exp Med. 1967 Oct 1;126(4):727–743. doi: 10.1084/jem.126.4.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Toole C. M., Davies A. J. Pre-emption in immunity. Nature. 1971 Mar 19;230(5290):187–189. doi: 10.1038/230187a0. [DOI] [PubMed] [Google Scholar]
- Paul W. E., Katz D. H., Benacerraf B. Augmented anti-S 3 antibody responses to an S 3 -protein conjugate. J Immunol. 1971 Sep;107(3):685–688. [PubMed] [Google Scholar]
- Paul W. E., Siskind G. W., Benacerraf B., Ovary Z. Secondary antibody responses in haptenic systems: cell population selection by antigen. J Immunol. 1967 Oct;99(4):760–770. [PubMed] [Google Scholar]
- Pincus J. H., Haber E., Katz M., Pappenheimer A. M., Jr Antibodies to pneumococcal polysaccharides: relation between binding and electrophoretic heterogeneity. Science. 1968 Nov 8;162(3854):667–668. doi: 10.1126/science.162.3854.667. [DOI] [PubMed] [Google Scholar]
- Priori E. S., Dmochowski L., Myers B., Wilbur J. R. Constant production of type C virus particles in a continuous tissue culture derived from pleural effusion cells of a lymphoma patient. Nat New Biol. 1971 Jul 14;232(28):61–62. doi: 10.1038/newbio232061a0. [DOI] [PubMed] [Google Scholar]
- Radovich J., Talmage D. W. Antigenic competition: cellular or humoral. Science. 1967 Oct 27;158(3800):512–514. doi: 10.1126/science.158.3800.512. [DOI] [PubMed] [Google Scholar]
- SEVER J. L. Application of a microtechnique to viral serological investigations. J Immunol. 1962 Mar;88:320–329. [PubMed] [Google Scholar]
- Scott D. W., Gershon R. K. Determination of total and merecaptothanol-resistant antibody in the same serum sample. Clin Exp Immunol. 1970 Feb;6(2):313–316. [PMC free article] [PubMed] [Google Scholar]
- Siskind G. W., Paul W. E., Benacerraf B. Studies on the effect of the carrier molecule on antihapten antibody synthesis. I. Effect of carrier on the nature of the antibody synthesized. J Exp Med. 1966 Apr 1;123(4):673–688. doi: 10.1084/jem.123.4.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner L. A., Eisen H. N. The relative affinity of antibodies synthesized in the secondary response. J Exp Med. 1967 Dec 1;126(6):1185–1205. doi: 10.1084/jem.126.6.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theis G. A., Siskind G. W. Selection of cell populations in induction of tolerance: affinity of antibody formed in partially tolerant rabbits. J Immunol. 1968 Jan;100(1):138–141. [PubMed] [Google Scholar]
- Underdown B. J., Eisen H. N. Cross-reactions between 2,4-dinitrophenyl and 5-acetouracil groups. J Immunol. 1971 Jun;106(6):1431–1440. [PubMed] [Google Scholar]


