Abstract
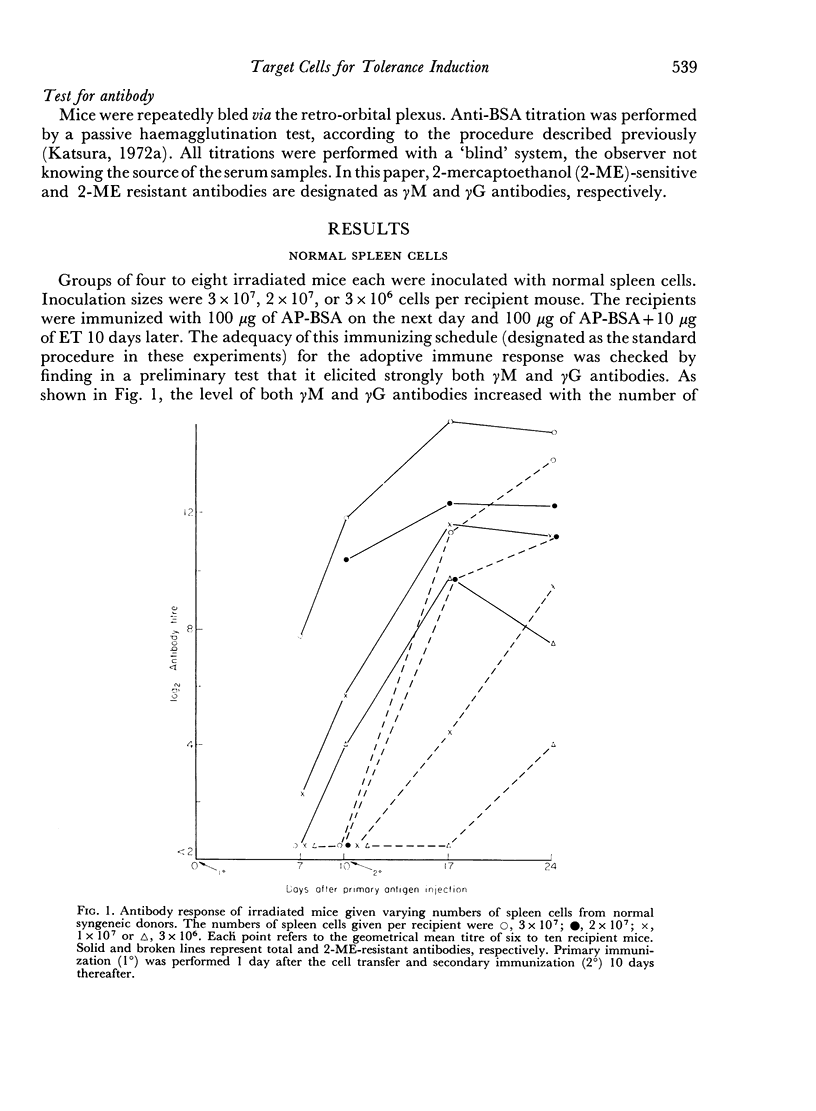
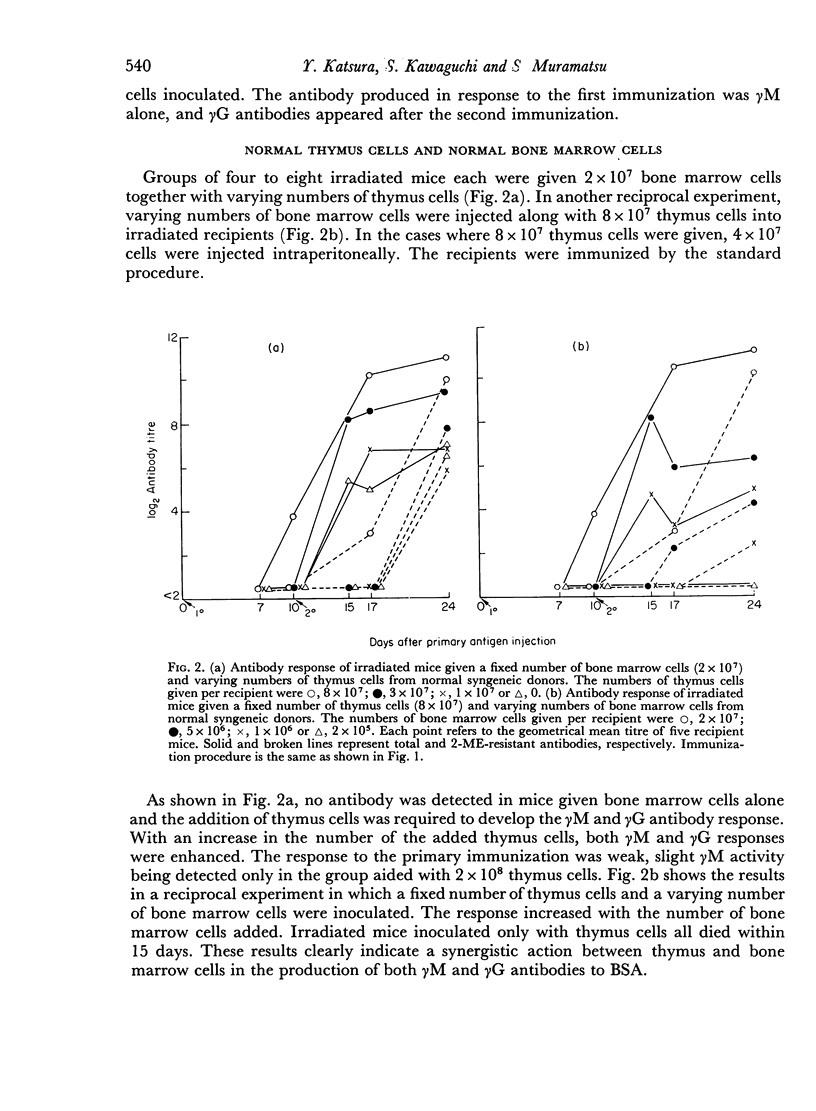
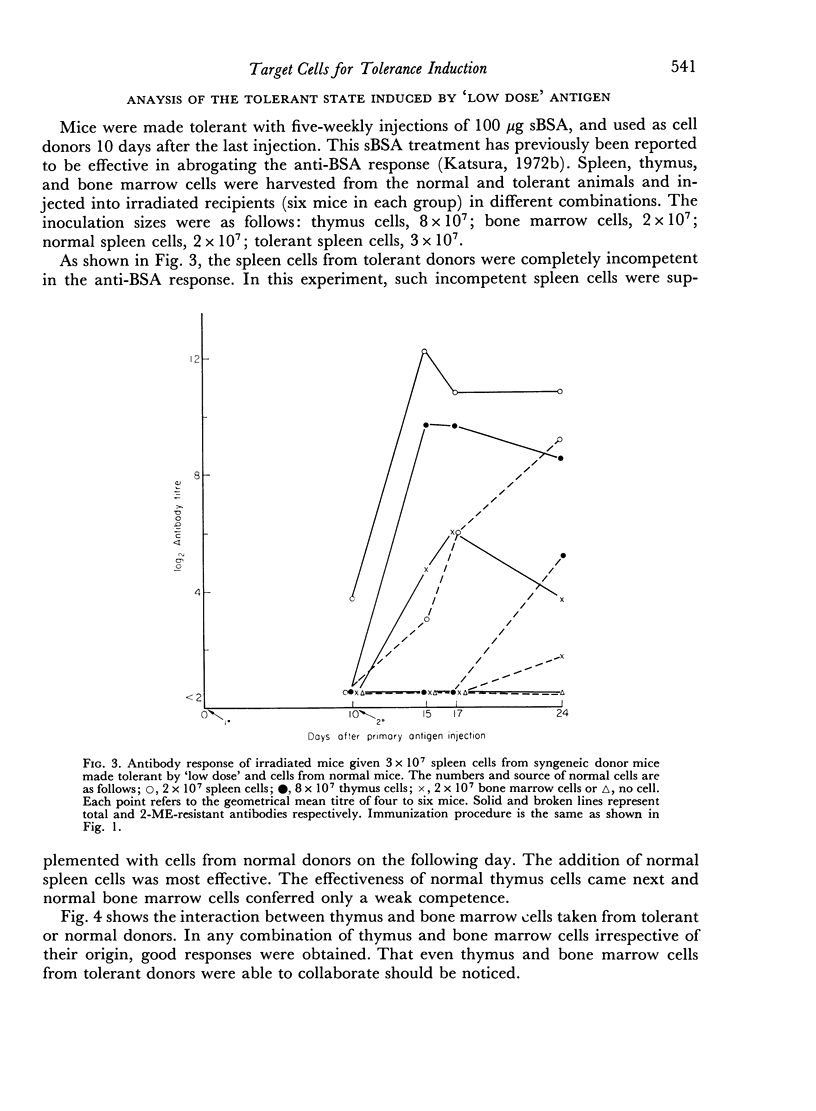
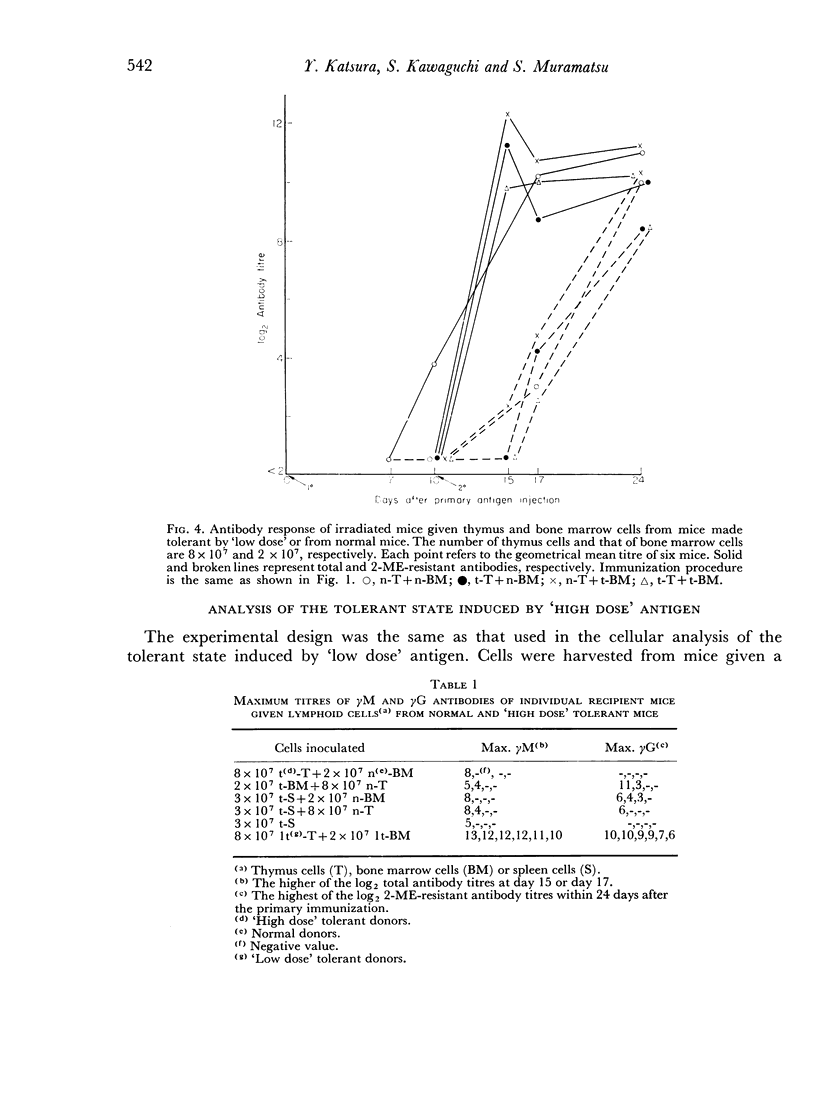
Bone marrow cells and thymus cells were observed in cell transfer experiments to collaborate in the production of anti-BSA antibodies. The target cells for tolerance induction either with a `low dose' of antigen (100 μg of deaggregated BSA once a week × 5) or with a `high dose' (a single injection of 5000 μg) were identified by the same technique. In `low dose' tolerance, some indication was obtained that thymus-derived cells in peripheral lymphoid systems were the target cells; bone marrow-derived cells appeared not to be so susceptible, and the cells residing in thymus or bone marrow seemed to remain unimpaired. In contrast, the injection of a `high dose' of tolerogen rendered both types of cells in spleen, thymus cells and bone marrow cells, unresponsive or hyporesponsive in parallel with one another.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Argyris B. F. Adoptive tolerance transferred by bone marrow, spleen, lymph node or thymus cells. J Immunol. 1966 Feb;96(2):273–278. [PubMed] [Google Scholar]
- Chiller J. M., Habicht G. S., Weigle W. O. Cellular sites of immunologic unresponsiveness. Proc Natl Acad Sci U S A. 1970 Mar;65(3):551–556. doi: 10.1073/pnas.65.3.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiller J. M., Habicht G. S., Weigle W. O. Kinetic differences in unresponsiveness of thymus and bone marrow cells. Science. 1971 Feb 26;171(3973):813–815. doi: 10.1126/science.171.3973.813. [DOI] [PubMed] [Google Scholar]
- Claman H. N., Chaperon E. A. Immunologic complementation between thymus and marrow cells--a model for the two-cell theory of immunocompetence. Transplant Rev. 1969;1:92–113. doi: 10.1111/j.1600-065x.1969.tb00137.x. [DOI] [PubMed] [Google Scholar]
- Claman H. N., Chaperon E. A., Triplett R. F. Thymus-marrow cell combinations. Synergism in antibody production. Proc Soc Exp Biol Med. 1966 Aug-Sep;122(4):1167–1171. doi: 10.3181/00379727-122-31353. [DOI] [PubMed] [Google Scholar]
- Isaković K., Smith S. B., Waksman B. H. Role of the thymus in tolerance. I. Tolerance to bovine gamma globulin in thymectomized, irradiated rats grafted with thymus from tolerant donors. J Exp Med. 1965 Dec 1;122(6):1103–1123. doi: 10.1084/jem.122.6.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsura Y. Studies on M and G antibody response of mice to bovine serum albumin. I. A systematic survey of immunizing conditions. Jpn J Microbiol. 1972 May;16(3):223–232. doi: 10.1111/j.1348-0421.1972.tb00652.x. [DOI] [PubMed] [Google Scholar]
- MITCHISON N. A. INDUCTION OF IMMUNOLOGICAL PARALYSIS IN TWO ZONES OF DOSAGE. Proc R Soc Lond B Biol Sci. 1964 Dec 15;161:275–292. doi: 10.1098/rspb.1964.0093. [DOI] [PubMed] [Google Scholar]
- Miller H. C., Cudkowicz G. Density gradient separation of marrow cells restricted for antibody class. Science. 1971 Mar 5;171(3974):913–915. doi: 10.1126/science.171.3974.913. [DOI] [PubMed] [Google Scholar]
- Miller J. F., Mitchell G. F. Cell to cell interaction in the immune response. V. Target cells for tolerance induction. J Exp Med. 1970 Apr 1;131(4):675–699. doi: 10.1084/jem.131.4.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell G. F., Miller J. F. Cell to cell interaction in the immune response. II. The source of hemolysin-forming cells in irradiated mice given bone marrow and thymus or thoracic duct lymphocytes. J Exp Med. 1968 Oct 1;128(4):821–837. doi: 10.1084/jem.128.4.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchison N. A. The dosage requirements for immunological paralysis by soluble proteins. Immunology. 1968 Oct;15(4):509–530. [PMC free article] [PubMed] [Google Scholar]
- NEEPER C. A., SEASTONE C. V. MECHANISMS OF IMMUNOLOGIC PARALYSIS BY PNEUMOCOCCAL POLYSACCHARIDE. I. STUDIES OF ADOPTIVELY ACQUIRED IMMUNITY TO PNEUMOCOCCAL INFECTION IN IMMUNOLOGICALLY PARALYZED AND NORMAL MICE. J Immunol. 1963 Sep;91:374–377. [PubMed] [Google Scholar]
- Nossal G. J., Cunningham A., Mitchell G. F., Miller J. F. Cell to cell interaction in the immune response. 3. Chromosomal marker analysis of single antibody-forming cells in reconstituted, irradiated, or thymectomized mice. J Exp Med. 1968 Oct 1;128(4):839–853. doi: 10.1084/jem.128.4.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Playfair J. H. Specific tolerance to sheep erythrocytes in mouse bone marrow cells. Nature. 1969 May 31;222(5196):882–883. doi: 10.1038/222882a0. [DOI] [PubMed] [Google Scholar]
- Taylor R. B. Cellular cooperation in the antibody response of mice to two serum albumins: specific function of thymus cells. Transplant Rev. 1969;1:114–149. doi: 10.1111/j.1600-065x.1969.tb00138.x. [DOI] [PubMed] [Google Scholar]


