Abstract
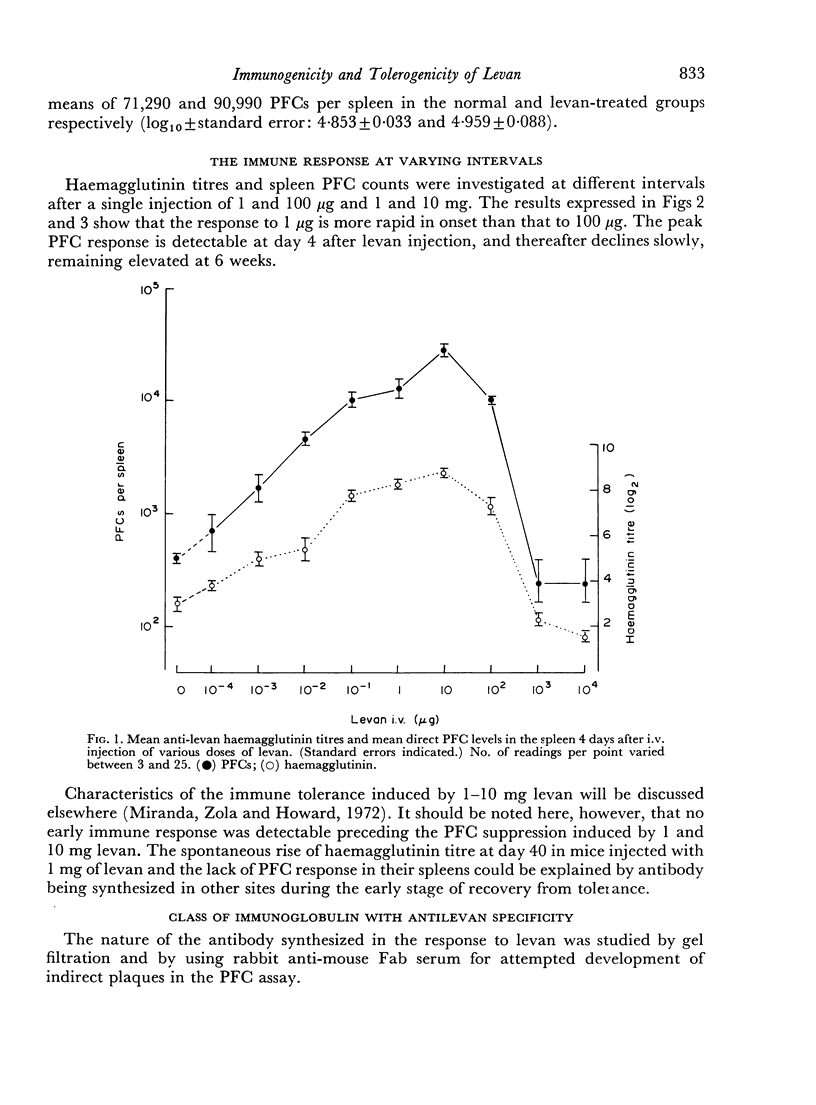
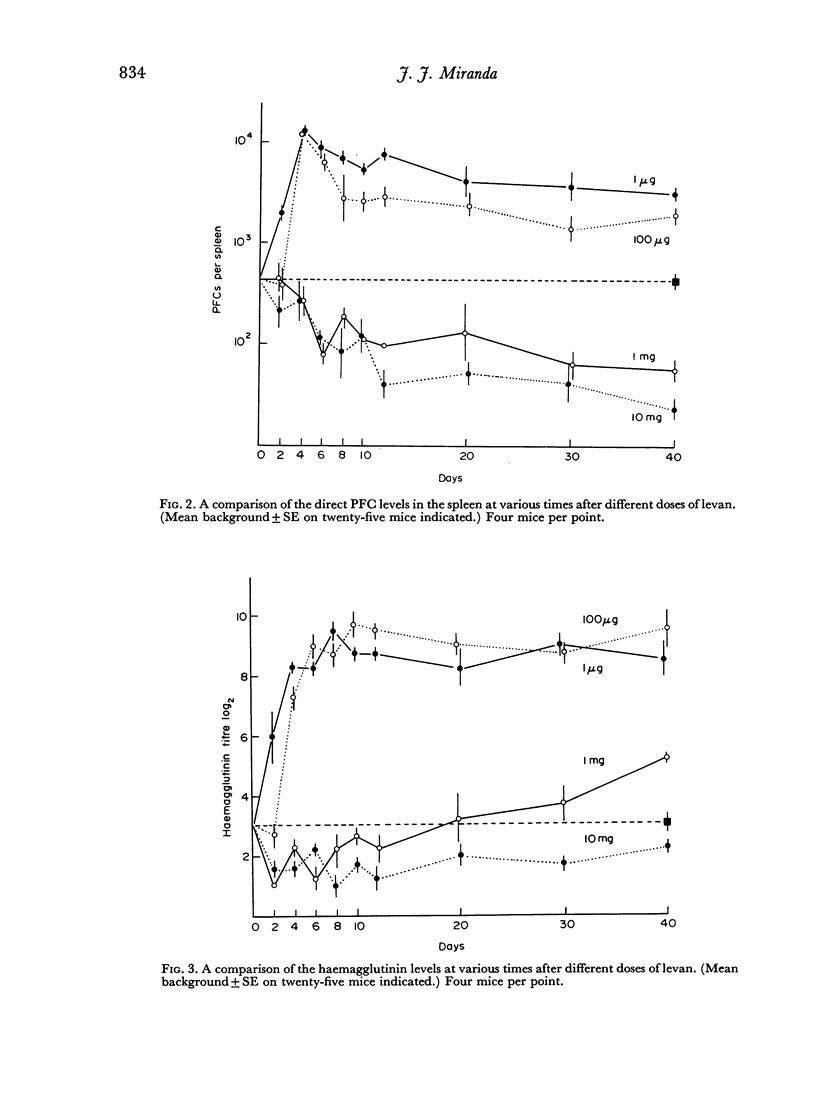
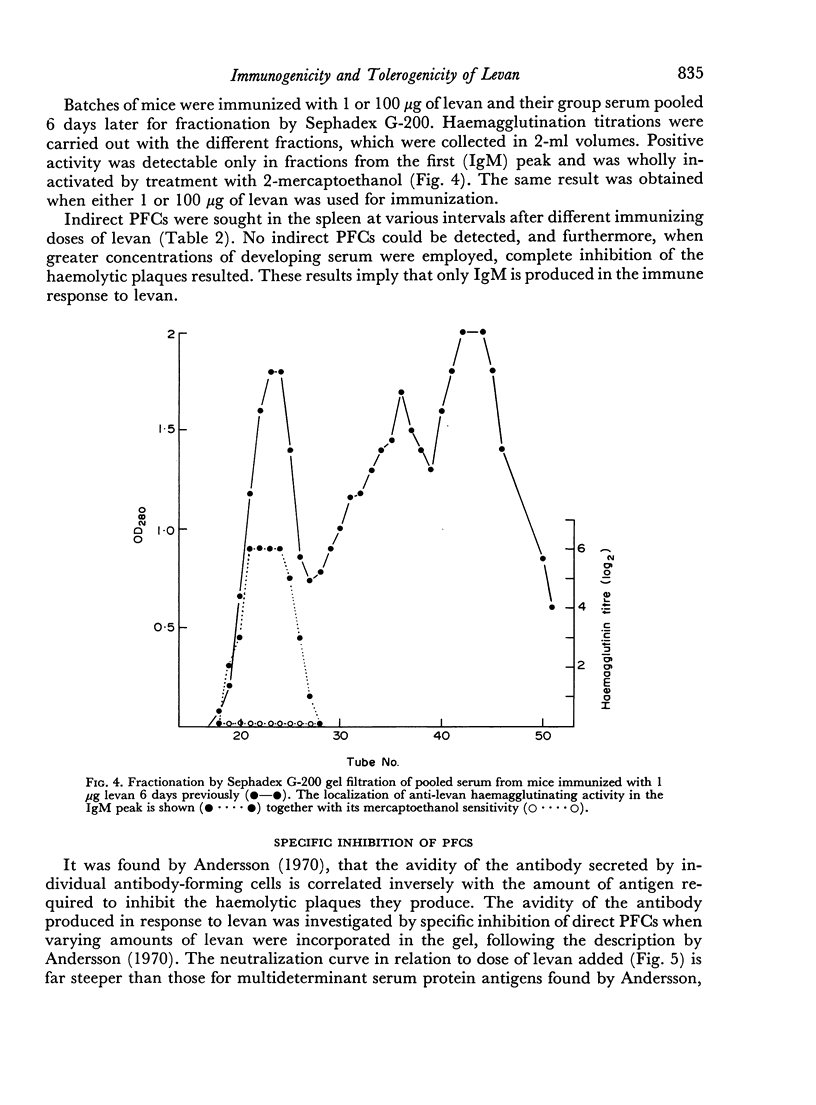
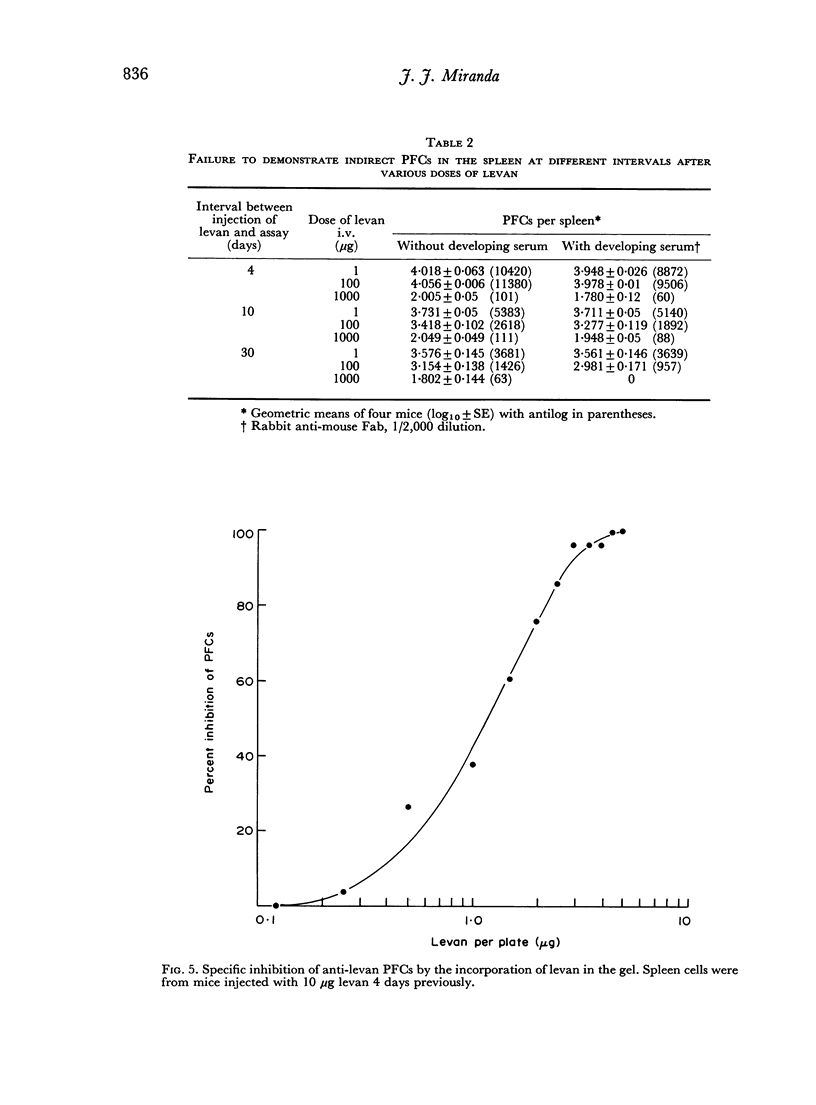
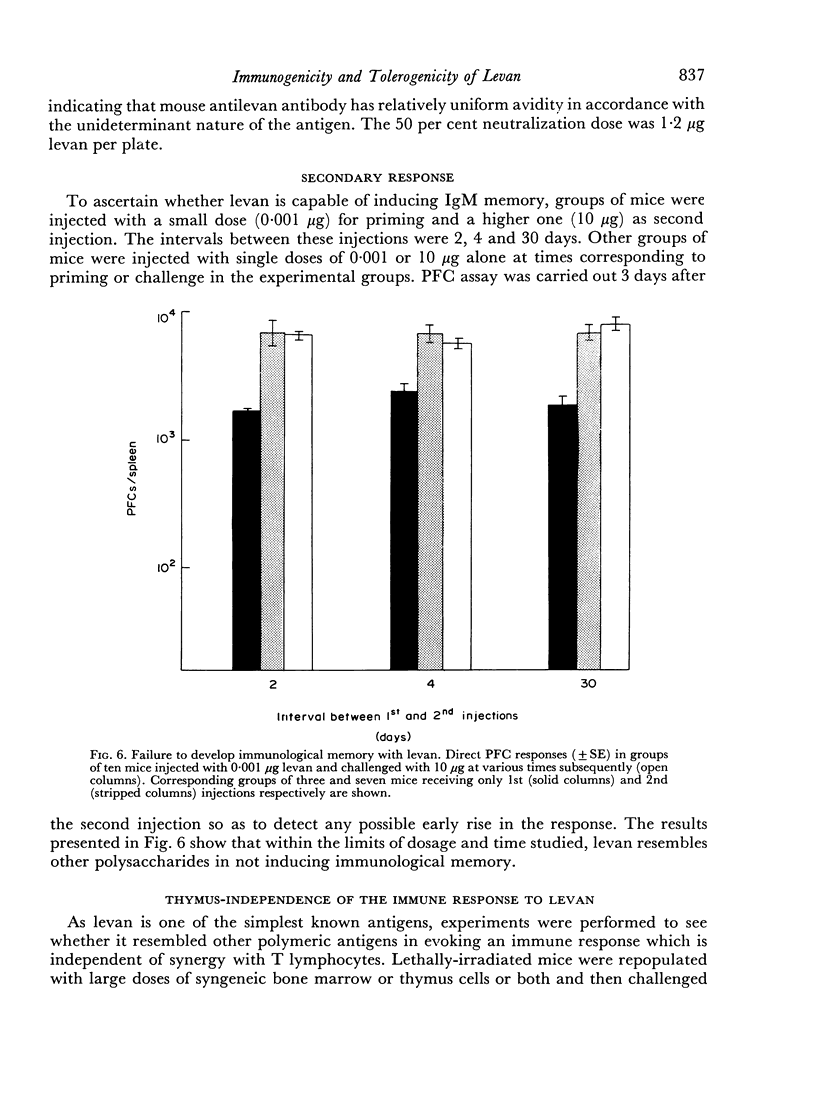
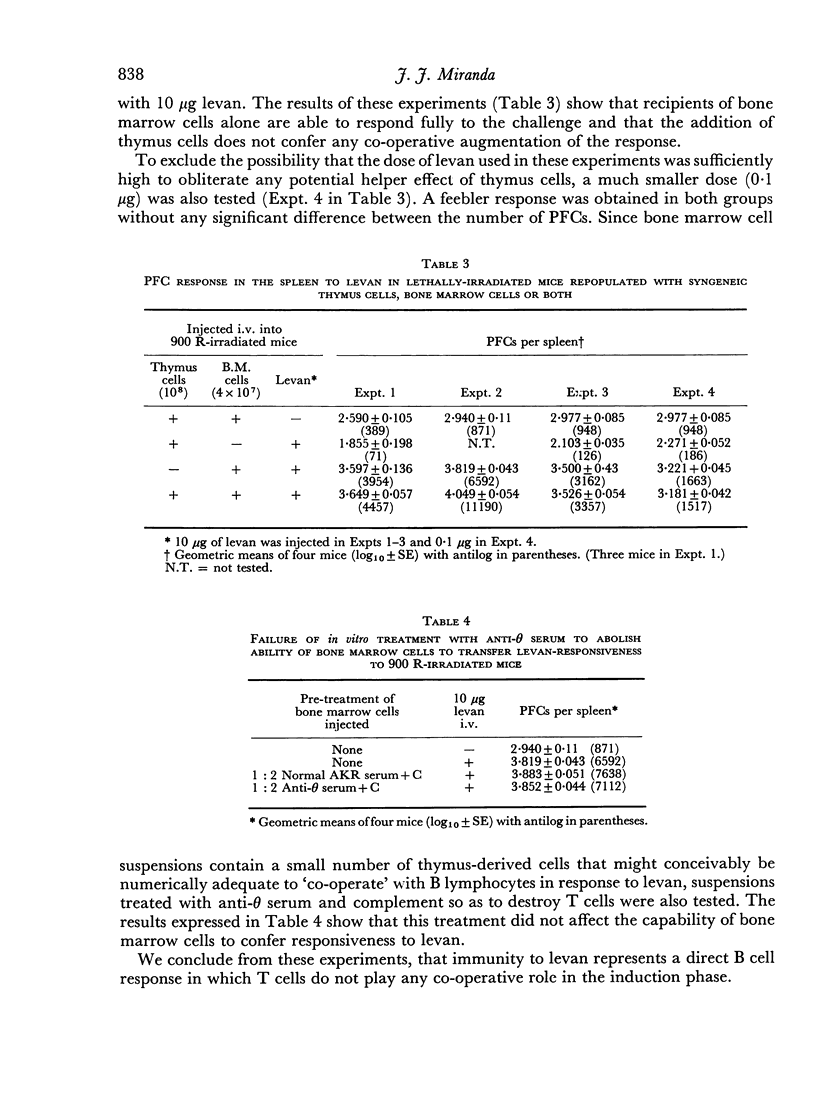
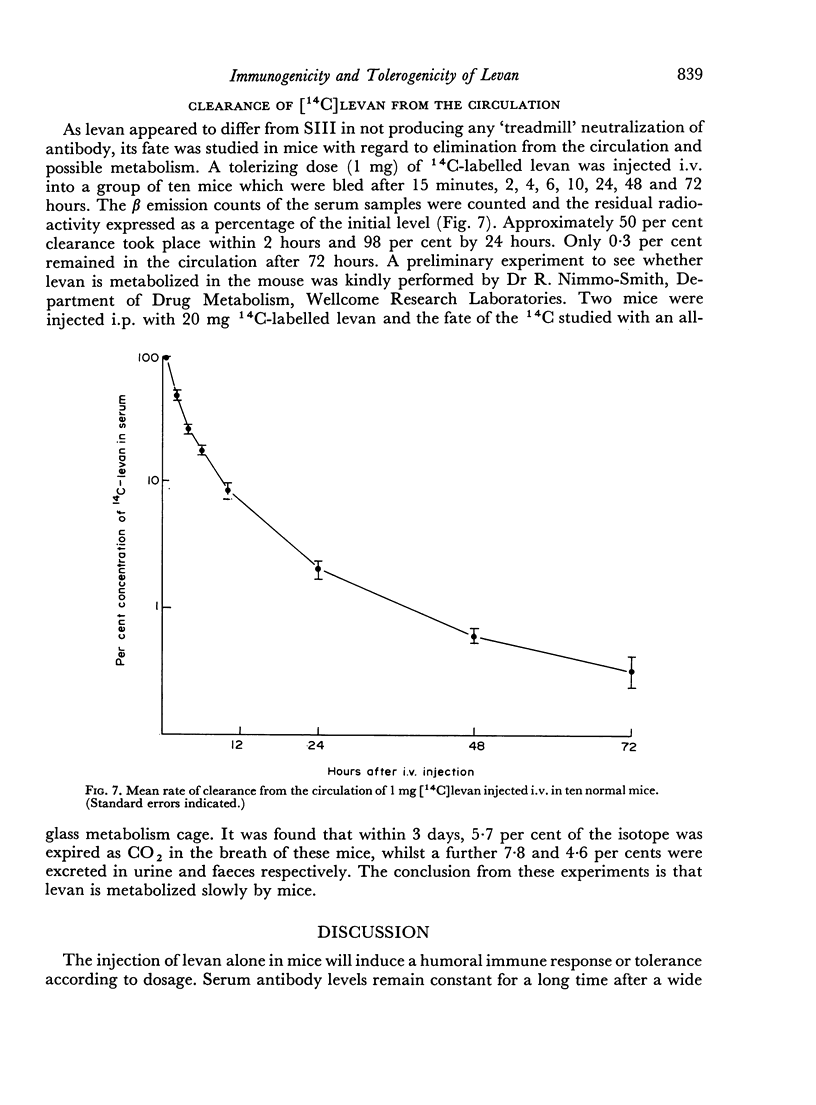
The immunological response to native levan (a fructose homopolymer with molecular weight 2×107) has been studied in (DBA/1×CBA-T6T6)F1 mice by passive haemagglutination and plaque-forming cell (PFC) assays. It resembles type-specific pneumococcal polysaccharide (SSS) in eliciting a prolonged humoral antibody response over a wide dose range (0.0001–100 μg) and in inducing longlasting `high zone' tolerance with a single injection (1 mg or more). Other similarities include an exclusively IgM response, independence of synergy with thymus-derived lymphocytes and absence of immunological memory. On the other hand, parallelism between serum haemagglutinin and PFC levels following all doses of antigen implies that higher immunizing doses of levan, unlike SSS, do not engage in peripheral neutralization of antibody. It was concluded from studying the fate of 14C-labelled levan that this was attributable to more rapid elimination from the circulation and subsequent slow metabolism of this polysaccharide. Levan also differs from SSS in inducing tolerance directly, without a detectable prior immune phase.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALLEN P. Z., KABAT E. A. Studies on the capacity of some polysaccharides to elicit antibody formation in man. J Exp Med. 1957 May 1;105(5):383–394. doi: 10.1084/jem.105.5.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson B., Blomgren H. Evidence for thymus-independent humoral antibody production in mice against polyvinylpyrrolidone and E. coli lipopolysaccharide. Cell Immunol. 1971 Oct;2(5):411–424. doi: 10.1016/0008-8749(71)90052-9. [DOI] [PubMed] [Google Scholar]
- Andersson B. Studies on the regulation of avidity at the level of the single antibody-forming cell. The effect of antigen dose and time after immunization. J Exp Med. 1970 Jul 1;132(1):77–88. doi: 10.1084/jem.132.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. J., Stashak P. W., Amsbaugh D. F., Prescott B. Characterization of the antibody response to type 3 pneumococcal polysaccharide at the cellular level. I. Dose-response studies and the effect of prior immunization on the magnitude of the antibody response. Immunology. 1971 Apr;20(4):469–480. [PMC free article] [PubMed] [Google Scholar]
- Bing D. H., Weyand J. G., Stavitsky A. B. Hemagglutination with aldehyde-fixed erythrocytes for assay of antigens and antibodies. Proc Soc Exp Biol Med. 1967 Apr;124(4):1166–1170. doi: 10.3181/00379727-124-31953. [DOI] [PubMed] [Google Scholar]
- Bretscher P., Cohn M. A theory of self-nonself discrimination. Science. 1970 Sep 11;169(3950):1042–1049. doi: 10.1126/science.169.3950.1042. [DOI] [PubMed] [Google Scholar]
- Britton S. Regulation of antibody synthesis against Escherichia coli endotoxin. II. Specificity, dose requirements and duration of paralysis induced in adult mice. Immunology. 1969 Apr;16(4):513–526. [PMC free article] [PubMed] [Google Scholar]
- Byfield P., Sercarz E. The X-Y-Z scheme of immunocyte maturation. VII. Cell division and the establishment of short-term IgM memory. J Exp Med. 1969 May 1;129(5):897–907. doi: 10.1084/jem.129.5.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiller J. M., Habicht G. S., Weigle W. O. Cellular sites of immunologic unresponsiveness. Proc Natl Acad Sci U S A. 1970 Mar;65(3):551–556. doi: 10.1073/pnas.65.3.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claman H. N., Chaperon E. A., Triplett R. F. Thymus-marrow cell combinations. Synergism in antibody production. Proc Soc Exp Biol Med. 1966 Aug-Sep;122(4):1167–1171. doi: 10.3181/00379727-122-31353. [DOI] [PubMed] [Google Scholar]
- Cunningham A. J. Studies on the cellular basis of IgM immunological memory. Immunology. 1969 May;16(5):621–632. [PMC free article] [PubMed] [Google Scholar]
- HUMPHREY J. H., PARROTT D. M., EAST J. STUDIES ON GLOBULIN AND ANTIBODY PRODUCTION IN MICE THYMECTOMIZED AT BIRTH. Immunology. 1964 Jul;7:419–439. [PMC free article] [PubMed] [Google Scholar]
- Hirata A. A., Brandriss M. W. Passive hemagglutination procedures for protein and polysaccharide antigens using erythrocytes stabilized by aldehydes. J Immunol. 1968 Mar;100(3):641–646. [PubMed] [Google Scholar]
- Howard J. G. Cellular events in the induction and loss of tolerance to pneumococcal polysaccharides. Transplant Rev. 1972;8:50–75. doi: 10.1111/j.1600-065x.1972.tb01564.x. [DOI] [PubMed] [Google Scholar]
- Howard J. G., Christie G. H., Courtenay B. M. Studies on immunological paralysis. IV. The relative contributions of continuous antibody neutralization and central inhibition to paralysis with type 3 pneumococcal polysaccharide. Proc R Soc Lond B Biol Sci. 1971 Sep 28;178(1053):417–438. doi: 10.1098/rspb.1971.0073. [DOI] [PubMed] [Google Scholar]
- Howard J. G., Christie G. H., Courtenay B. M. Studies on immunological paralysis. VII. Rapid reversal of Felton's paralysis as evidence for 'tolerant' cells. Proc R Soc Lond B Biol Sci. 1972 Mar 14;180(1060):347–361. doi: 10.1098/rspb.1972.0021. [DOI] [PubMed] [Google Scholar]
- Howard J. G., Christie G. H., Jacob M. J., Elson J. Studies on immunological paralysis. 3. Recirculation and antibody-neutralizing activity of 14C-labelled type 3 pneumococcal polysaccharide in paralysed mice. Clin Exp Immunol. 1970 Oct;7(4):583–596. [PMC free article] [PubMed] [Google Scholar]
- Hämmerling U., Westphal O. Synthesis and use of O-stearoyl polysaccharides in passive hemagglutination and hemolysis. Eur J Biochem. 1967 Mar;1(1):46–50. doi: 10.1007/978-3-662-25813-2_9. [DOI] [PubMed] [Google Scholar]
- Miranda J. J., Zola H., Howard J. G. Studies on immunological paralysis. X. Cellular characteristics of the induction and loss of tolerance to levan (Polyfructose). Immunology. 1972 Dec;23(6):843–855. [PMC free article] [PubMed] [Google Scholar]
- Mitchell G. F., Miller J. F. Cell to cell interaction in the immune response. II. The source of hemolysin-forming cells in irradiated mice given bone marrow and thymus or thoracic duct lymphocytes. J Exp Med. 1968 Oct 1;128(4):821–837. doi: 10.1084/jem.128.4.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Möller G. Immunocyte triggering. Cell Immunol. 1970 Dec;1(6):573–582. doi: 10.1016/0008-8749(70)90023-7. [DOI] [PubMed] [Google Scholar]
- Nossal G. J., Austin C. M., Ada G. L. Antigens in immunity. VII. Analysis of immunological memory. Immunology. 1965 Oct;9(4):333–348. [PMC free article] [PubMed] [Google Scholar]
- Raff M. C. Role of thymus-derived lymphocytes in the secondary humoral immune response in mice. Nature. 1970 Jun 27;226(5252):1257–1258. doi: 10.1038/2261257a0. [DOI] [PubMed] [Google Scholar]
- Sercarz E. E., Byers V. S. The X-Y-Z scheme of immunocyte maturation. 3. Early IgM memory and the nature of the memory cell. J Immunol. 1967 Apr;98(4):836–843. [PubMed] [Google Scholar]
- Siskind G. W., Howard J. G. Studies on the induction of immunological unresponsiveness to pneumococcal polysaccharide in mice. J Exp Med. 1966 Sep 1;124(3):417–429. doi: 10.1084/jem.124.3.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor R. B., Wortis H. H. Thymus dependence of antibody response: variation with dose of antigen and class of antibody. Nature. 1968 Nov 30;220(5170):927–928. doi: 10.1038/220927a0. [DOI] [PubMed] [Google Scholar]
- Unanue E. R. Thymus dependency of the immune response to hemocyanin: an evaluation of the role of macrophages in thymectomized mice. J Immunol. 1970 Dec;105(6):1339–1343. [PubMed] [Google Scholar]


