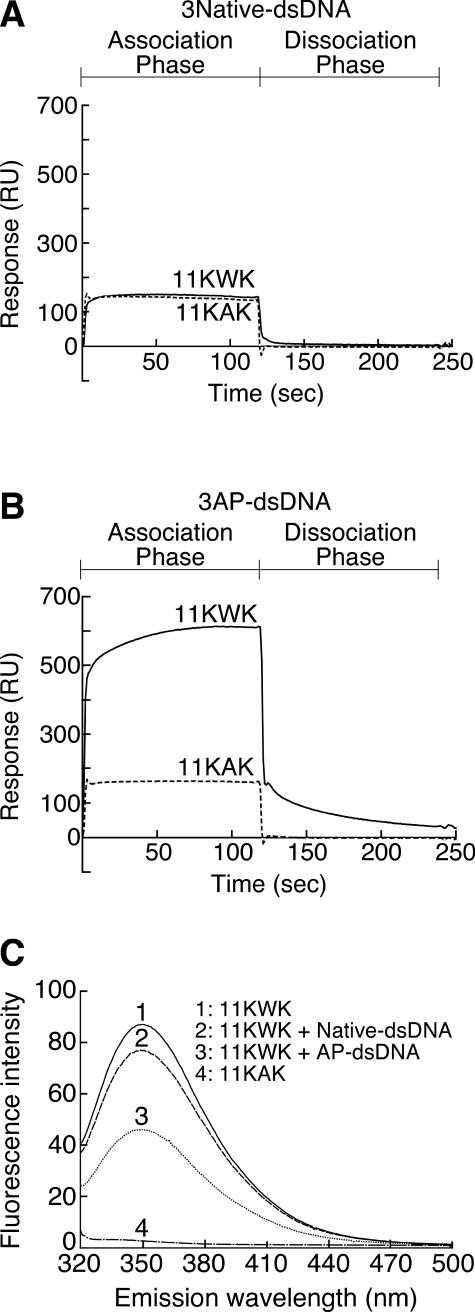
Figure 7.
Effect of tryptophan residues on interaction between peptides and double-stranded oligonucleotides containing AP sites or not. (A) SPR analyses of the interaction between a peptide containing a tryptophan residue (11KWK, solid line) or not (11KAK, dashed line) and dsDNA not containing an AP site. The amount of immobilized oligonucleotides not containing AP sites (3Native-dsDNA) was 1515 RU. For all the sensorgrams, the injection point was set as the zero time and the baseline prior to the injection was set to zero RU. The 11KWK and 11KAK oligopeptides were KSRGKWKGRSA and KSRGKAKGRSK, respectively. (B) SPR analyses of the interaction between a peptide containing a tryptophan residue (11KWK, solid line) or not (11KAK, dashed line) and dsDNA containing three AP sites. The amount of immobilized oligonucleotide containing three AP sites (3AP-dsDNA) was 1500 RU. This analysis was carried out under the same experimental conditions as in (A), except for the immobilized oligonucleotide. (C) Fluorescence spectra of the peptide containing a tryptophan residue in the presence or absence of dsDNA containing an AP site. AP-dsDNA was the substrate used in the AP endonuclease assay (Figure 2). The DNA sequences of AP-dsDNA and Native-dsDNA were the same excluding the presence of an AP site. Peptide and dsDNA were mixed at a molar ratio of 1:10. The excitation wavelength was 275 nm.