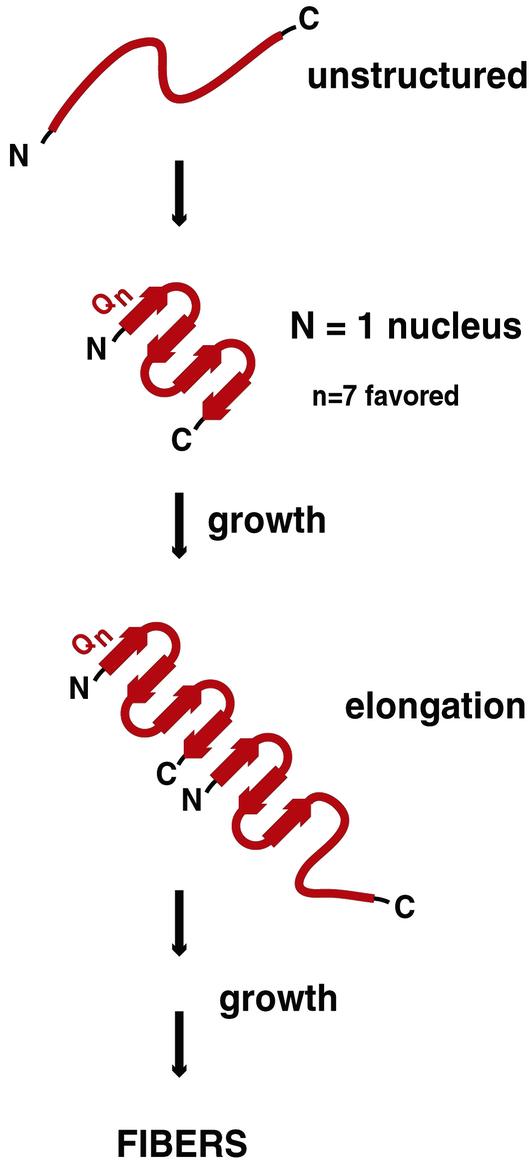
Figure 2.
Model of polyGln aggregate initiation and elongation as proposed by Thakur and Wetzel (30). Before the conformational change that initiates disease pathogenesis, mutant polyGln lacks secondary structure. A polyGln monomer undergoes a structural transition to a four-stranded antiparallel β-sheet, with an optimum of seven glutamine residues per β-strand (extended chain). This structured monomer serves as a nucleus for binding of a second unstructured monomer. Binding of the disordered monomer to the ordered nucleus results in acquisition of β-structure in the newly added monomer, providing a new elongation site, and is referred to as template-assisted or “dock-and-lock” elongation. Adapted from Chen et al. (28).