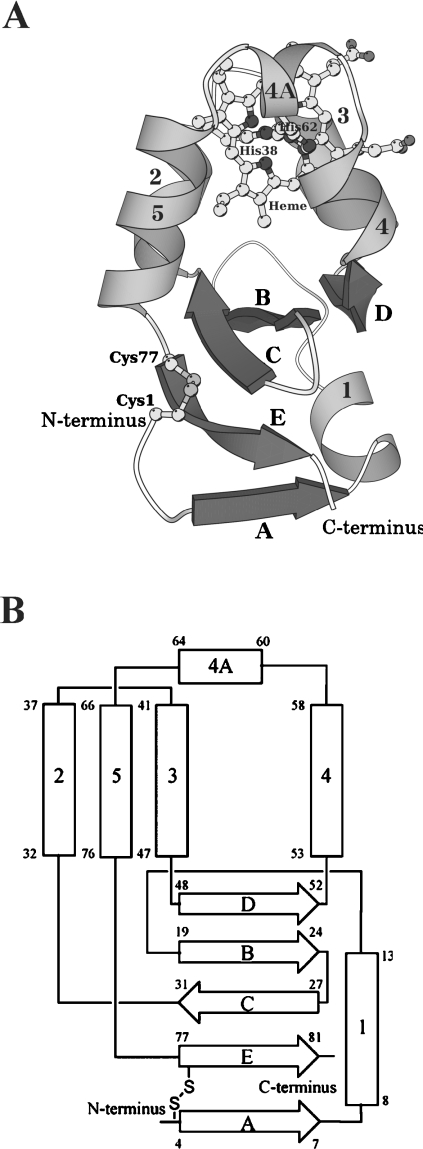
Figure 1. Overall structure (A) and topology diagram (B) of A. suum cytochrome b5.
The protein is folded in six helices and five β strands, comprising a β-pleated sheet. (A) Ribbon and ball-and-stick drawing of the overall structure. The six helices are numbered 1, 2, 3, 4, 4A and 5 in grey, while the five β strands are denoted A–E in dark grey. The haem and His38, His62, Cys1 and Cys77 are drawn as ball-and-stick models. (B) α-Helices and β-strands are represented as rectangles and broad arrows respectively, with residue numbers showing their starting and ending positions. Two histidine residues (His38 and His62) ligating the haem iron and the disulphide bond between Cys1 and Cys77 are also shown. The nomenclature of α-helices and β-strands is the same as in (A).