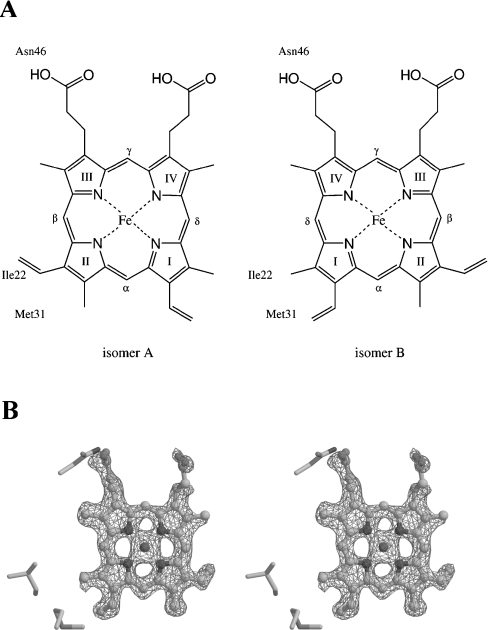
Figure 3. Haem conformation in A. suum cytochrome b5.
(A) Two haem conformations about the α,γ-meso axis are denoted as isomers A and B. The haem-Fe atom is bound to His38 and His62 from the front and back sides respectively. Three residues, Ile22, Met31 and Asn46, which are in close contact with the haem group, are shown in the respective Figure. (B) Stereo view of an Fo-Fc omit map for haem at 1.8 Å resolution. The haem group and residues within 10 Å of the haem are excluded from structure factor calculations and difference electron density is contoured at the 3σ level. Isomer B, the predominant haem form of A. suum cytochrome b5, is drawn as a ball-and-stick model.