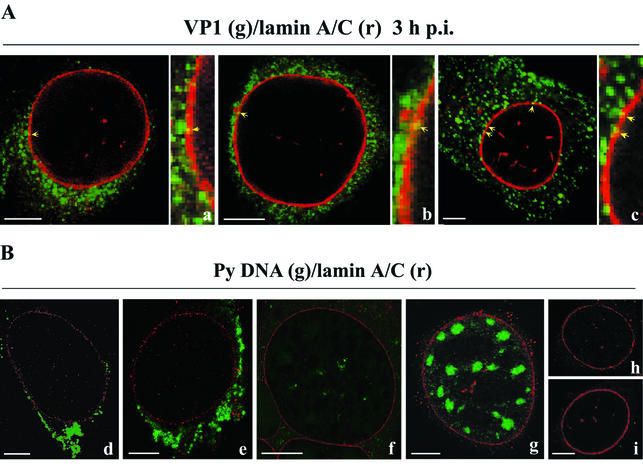
FIG. 7.
(A) Immunolocalization of VP1 (green) and lamin A/C (red). 3T6 cells were infected with mouse polyomavirus ([a and b] multiplicity of infection, 5 × 102 PFU per cell; [c] multiplicity of infection, 102 PFU per cell) and fixed at 3 h postinfection. Indirect immunofluorescent staining of VP1 (mouse polyclonal anti-VP1 antibody followed by Alexa Fluor-488 rabbit anti-mouse immunoglobulin) and lamin A/C (goat polyclonal anti-lamin A/C antibody followed by Alexa Fluor-546 donkey anti-goat immunoglobulin) was performed, and merged confocal sections are shown. Bars, 5 μm. (B) Localization of polyomavirus DNA (green) and lamin A/C (red). 3T6 cells were infected with mouse polyomavirus (Py) ([d and e] multiplicity of infection, 5 × 102 PFU per cell; [f and g] multiplicity of infection, 102 PFU per cell). Cells were fixed at 6 h postinfection (d and e), 10 h postinfection (f), or 15 h postinfection (g), and polyomavirus DNA was detected by in situ hybridization with the Alexa Fluor-488-5-dUTP-stained random-primed probes against the polyomavirus genome (see text). Indirect immunofluorescence staining of lamin A/C (goat polyclonal anti-lamin A/C antibody followed by Alexa Fluor-546 donkey anti-goat immunoglobulin) was performed after in situ hybridization. Merged confocal sections are shown. As a negative control, in situ hybridization and immunostaining of lamin A/C on noninfected 3T6 cells were done (h and i). Bars, 5 μm.