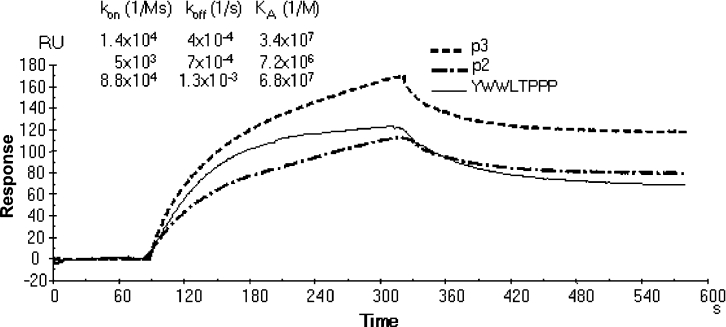
Figure 8. Kinetics of PA63 binding.
Biotinylated monomeric peptides, diluted to 100 μg/ml in HBS, were immobilized on streptavidin-coated SA sensor chip at a flow rate of 10 μl/min. For kinetic experiments, serial dilutions of PA63 in HBS at concentrations ranging from 1 μM to 10 nM, were injected at a flow rate of 10 μl/min over immobilized peptides. Association and dissociation kinetic rate constants (kon and koff) and the equilibrium association constant Ka were calculated using BIAevaluation 3.0 software. The overlay of sensorgrams from BIAcore analysis of PA63 (300 nM) binding to biotinylated monomeric peptides bound to an SA sensor chip is shown.