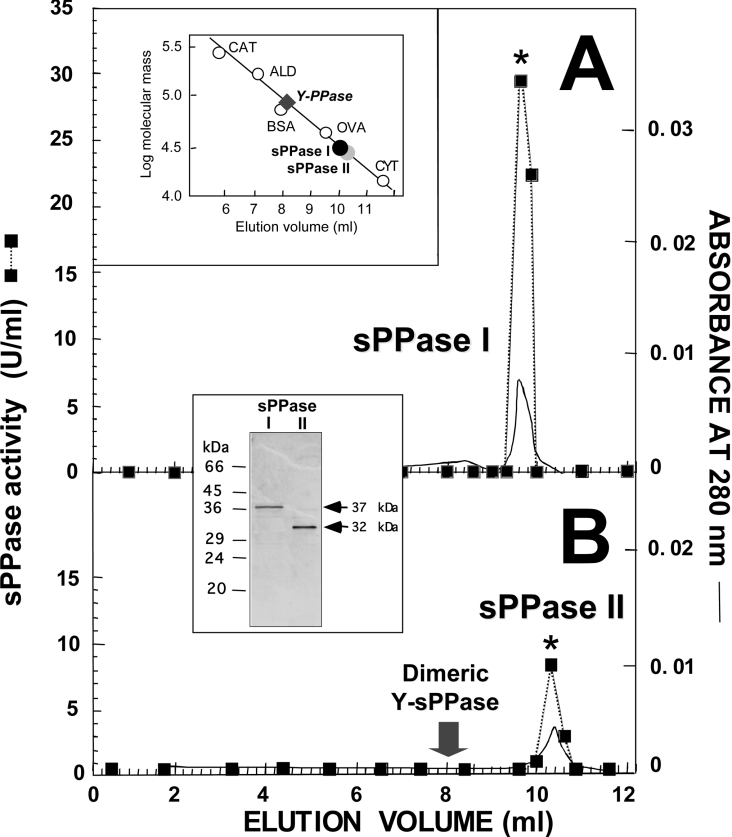
Figure 2. FPLC gel-filtration chromatography on Superose 12HR column of the sPPase isoforms co-purified from Ch. reinhardtii.
Aliquots (0.5 ml) of Cr-sPPase I (approx. 70 μg) (A) and Cr-sPPase II (approx. 35 μg) (B) purified preparations, previously resolved using this technique, were applied on to a Superose 12HR 10/30 column. Isocratic elution was achieved at a flow rate of 1 ml/min and 0.2 ml fractions were collected. The upper inset shows the calibration of the column with protein standards (CAT, catalase; ALD, aldolase; OVA, ovoalbumin; CYT, cytochrome c) and the positions of the sPPase peaks, which correspond to native molecular masses of approx. 33 kDa (black circle, Cr-sPPase I) and approx. 28 kDa (grey circle, Cr-sPPase II). The Coomassie-Blue-stained SDS/PAGE gel (lower inset; approx. 4 μg protein per lane) shows the single 37 kDa and 32 kDa protein bands present in the fractions with highest activity (*), indicating that the proteins are monomers. The positions and molecular masses of protein standards are indicated on the left-hand side of the SDS/PAGE gel. The elution position of a commercial cytosolic Y-sPPase is consistent with a dimeric structure (approx. 70 kDa), indicated for comparison by an arrow in (B) and by a diamond in the upper inset.