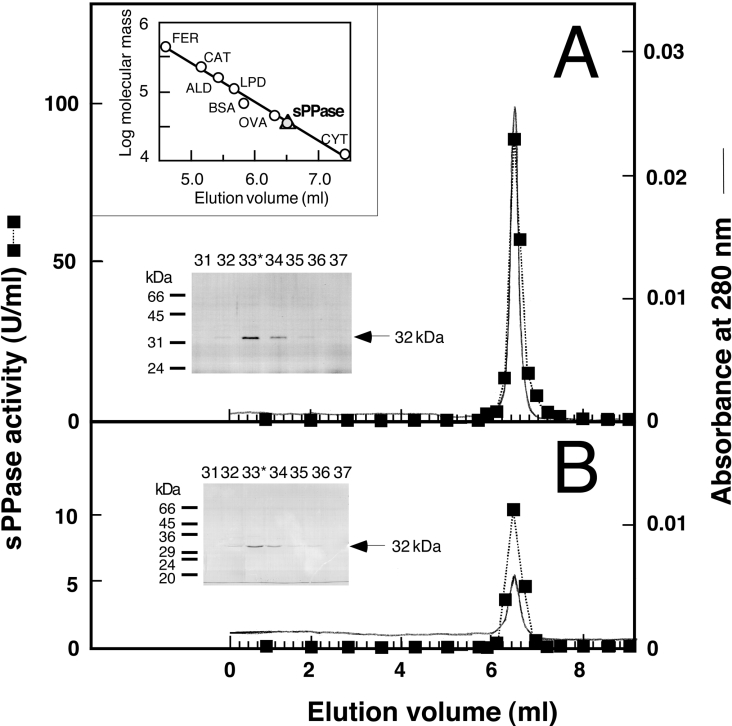
Figure 3. FPLC gel-filtration chromatography on Superose 12HR column of the sPPase purified from whole cells and cyanelles of Cy. paradoxa.
Aliquots (0.1 ml) of sPPase preparations (hydroxyapatite eluate) purified from whole cells (approx. 150 μg) (A) or isolated cyanelles (approx. 50 μg) (B) were applied on to a Superose 12HR 10/30 column. Elution (0.4 ml/min) and fractions were collected as described in Figure 2. The upper inset shows the calibration of the column with protein standards (FER, ferritine; CAT, catalase; ALD, aldolase; LPD, lipoamide dehydrogenase; OVA, ovoalbumin; CYT, cytochrome c) and the position of sPPase peaks (triangle and grey circle) that corresponds to a native molecular mass of approx. 33 kDa. The Coomassie-Blue-stained SDS/PAGE gels of the indicated fractions around the activity peak (*, highest activity fraction) show a single 32 kDa protein (arrows) that co-eluted with sPPase activity in both cases. The positions and molecular masses of protein standards are indicated on the left-hand side of the SDS/PAGE gels.