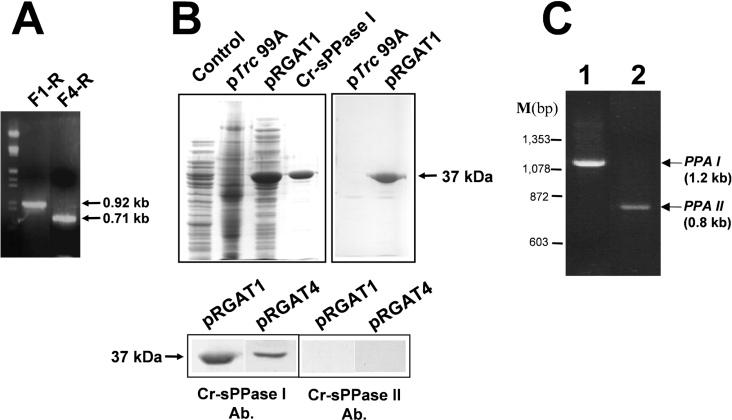
Figure 7. Cloning of two A. thaliana cDNA amplicons encoding a chloroplastic sPPase putative precursor and its predicted mature protein, and immunochemical characterization of the corresponding recombinant proteins produced in E. coli.
(A) Electrophoresis on 0.7% agarose gel of the single PCR bands amplified with specific primer pairs F1-R (0.92 kb, complete sPPase I precursor ORF) and F2-R (0.71 kb, predicted sPPase I mature protein ORF). Both ORFs correspond to the A. thaliana gene AT5G09650. Lambda phage DNA cleaved with HindIII and EcoRI was used as a bp marker. (B) Upper panel: Coomassie-Blue-stained SDS/PAGE (left-hand panel) of cell-free extracts (50 μg of protein per lane) from E. coli XL1blue transformed with empty pTrc99A (control), transformed with pRGAT1 (F1-R cDNA construct) expressing a 37 kDa protein and 5 μg of purified Cr-sPPase I, and immunodetection (right-hand panel) of the recombinant plant sPPase I-like protein by the anti-(Cr-sPPase I) antibody. Lower panel: immunodetection of over-expressed plant 37 kDa protein with the anti-(Cr-sPPase I) antibody in cell-free extracts of E. coli XL1blue clones (40 μg of protein per lane) transformed with pRGAT1 (F1-R cDNA construct, sPPase I chloroplast precursor ORF) and pRGAT4 (F4-R cDNA construct, predicted sPPase I mature protein ORF). Note that no equivalent protein band was recognized by the anti-(Cr-sPPase II) antibody. (C) Electrophoretic analysis (0.7% agarose gel) of PCR-amplified Ch. reinhardtii cDNAs corresponding to the two different PPA genes encoding putative sPPases identified in EST databases. Single cDNA bands of the expected size for PPAI (1.2 kb, lane 1) and PPAII (0.8 kb, lane 2) cDNAs were obtained using a total cDNA preparation from Ch. reinhardtii cells as a template. ΦX174 phage DNA cleaved with HaeIII was used as a bp marker (M).