Figure 3.
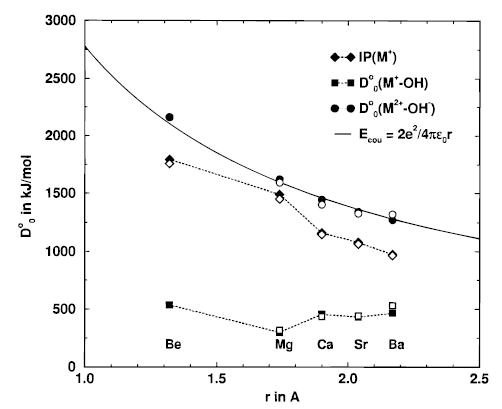
Experimental (open symbols) and calculated (filled symbols) values of IP(M+), D°0(M+–OH), and D°0(M2+–OH−) that are used in the thermochemical cycles (eqs 3 and 3’), as a function of the M–O1 distance in MOH+. The value of D°0(M2+–OH−) smoothly follows the Coulomb energy of a double and a single charge at distance r (solid line).
