Abstract
In the analysis of memory it is commonly observed that, even after a memory is apparently forgotten, its latent presence can still be revealed in a subsequent learning task. Although well established on a behavioral level, the mechanisms underlying latent memory are not well understood. To begin to explore these mechanisms, we have used Aplysia, a model system that permits the simultaneous study of memory at the behavioral, cellular, and molecular levels. We first demonstrate that robust latent memory is induced by long-term sensitization training of the tail-elicited siphon withdrawal reflex. It is revealed by its ability to facilitate the subsequent induction of three mechanistically distinct temporal domains of sensitization memory: short-term, intermediate-term, and long-term memory. Under our training conditions, the latent memory persists for at least 2 d following the decay of original memory expression but appears to be gone by 4 d. Interestingly, we also find that latent memory is induced even in the absence of overt memory for the original training. These findings now permit the analysis of the cellular and molecular architecture of a common feature of learning and memory.
A wide range of studies examining memory have revealed that, even after apparent forgetting, a latent (residual) memory persists and can be revealed by facilitated acquisition in a subsequent learning task. Often, this phenomenon is referred to as “savings.” The general notion of savings, as well as the empirical means of assessing it, was described more than a century ago by Ebbinghaus (1885/1913). Following this early insight into memory, many studies have revealed savings in a wide variety of learning tasks. A notable extension of this idea is “latent learning,” which refers to the well established phenomenon that latent memory can develop in the absence of apparent original learning (Tolman and Honzik 1930). Following these early seminal studies, various forms of savings and latent learning have been described in a large variety of animals, including humans (Lubow and Moore 1959; Nelson 1971; Plotkin and Oakley 1975; Nelson 1978; MacLeod 1988; Matzel et al. 1992; Lubow and Gewirtz 1995; Monk et al. 1996; Medina et al. 2001; Nicholson et al. 2003). Collectively these studies show that the general phenomenon of latent memory is a common feature of learning and memory across the animal kingdom.
While well established on a behavioral level, the cellular mechanisms underlying savings are not well understood. Such a mechanistic analysis requires a preparation that exhibits a robust form of latent memory in a system that is amenable to a cellular and molecular analysis. In the present paper we show that the marine mollusk Aplysia provides such a system. It is already well established that Aplysia demonstrates memory for sensitization of tail-elicited siphon withdrawal (T-SWR) in three mechanistically distinct temporal domains, short-term memory (STM), intermediate-term memory (ITM), and long-term memory (LTM) (Scholz and Byrne 1987; Castellucci et al. 1989; Goldsmith and Byrne 1993; Sutton et al. 2001, 2002). Here we show that sensitization training of the T-SWR induces a latent memory. Specifically, we show that the prior induction of LTM for sensitization facilitates the subsequent induction of memory, even after the original memory is apparently forgotten. Moreover, the latent memory formed with sensitization training facilitates the subsequent induction of memory in the short-term, intermediate-term, and long-term temporal domains. Finally, we find that latent memory can be induced even in the absence of apparent learning from the original sensitization training episode. The cellular and molecular mechanisms of memory for sensitization are relatively well understood in this system (Walters et al. 1983a, b; Scholz and Byrne 1987, 1988; Zhang et al. 1994; Cleary et al. 1998; Sutton and Carew 2000; Sutton et al. 2001; Sharma et al. 2003a, b; Sutton et al. 2004). Thus, it will now be possible to begin to explore the cellular and molecular mechanisms underlying latent memory.
Results
Sensitization training induces latent memory
We first asked whether sensitization training in Aplysia can induce memory that outlasts overt behavioral expression (latent memory). Our experimental strategy consisted of two phases. A schematic representation of the paradigm is shown in Figure 1. In Phase I, we induced LTM for sensitization of the T-SWR. We took advantage of recently described parameters for the induction of different temporal phases of sensitization memory within the T-SWR (Sutton et al. 2002), and modified these parameters to induce LTM of minimal duration. This optimization of initial forgetting allowed latent memory to be readily explored (see Discussion and Materials and Methods). At the end of Phase I training, we tracked memory retention in individual animals until they demonstrated clear forgetting. In Phase II, we assessed latent memory by retraining those animals which had previously expressed LTM for sensitization and subsequently exhibited two days of forgetting. Following retraining, we tested memory at 10 min, 2 h, and 24 h, compared with matched controls.
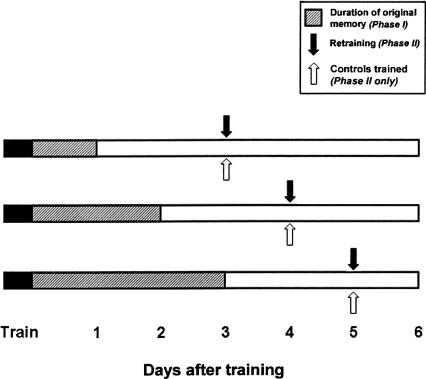
Figure 1.
Diagram of the experimental procedure. Experimental animals first received long-term sensitization training (solid black bars). Memory expression (hatched bars) was then monitored with two post-tests taken every 24 h (Phase I). On the second consecutive day without expression of the original memory (open bars), animals were retrained with two spaced tail shocks (Phase II, solid arrows). The duration of LTM expression in individual animals varied; thus, each experimental animal was retrained with a matched no-shock control (open arrows). Memory was assessed following retraining at 10 min, 2 h, and 24 h. Examples are given of individual cases where original memory expression lasted 1 d (top bar), 2 d (middle bar), or 3 d (bottom bar) and retraining occurred on the third, fourth, or fifth day, respectively, after original training.
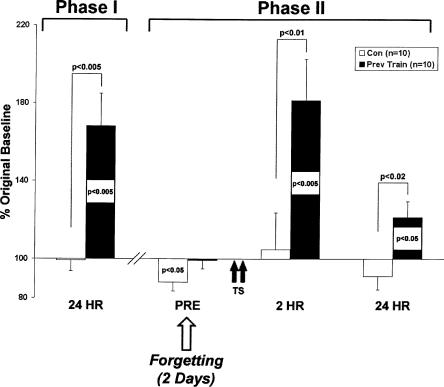
Prior to all experiments, we established the mean baseline T-SWR response for each animal by delivering three test stimuli to the posterior tail midline (0.5 s, inter-trial interval [ITI] = 15 min). All animals that demonstrated stable siphon withdrawal responses (>90% of animals, see Materials and Methods) were next divided randomly into control or experimental groups. In Phase I, experimental animals were given long-term sensitization training (see Materials and Methods). The resulting LTM for sensitization was assessed with two post-tests (ITI = 30 min) taken every 24 h. The mean of these two tests served as a composite retention score. Trained animals (n = 10) expressed significant LTM (mean ± SEM%, 168 ± 17%, P < 0.005) 24 h following training. In contrast, matched controls (n = 10, no shocks) demonstrated no LTM (99 ± 6%, NS, Fig. 2, Phase I). Moreover, trained animals showed significantly greater test responses than controls (P < 0.005). Memory expression in the trained animals lasted an average of 2 d.
Figure 2.
Sub-threshold training induces 2-h and 24-h memory in previously trained animals. Phase I: Trained animals (n = 10) exhibited robust LTM after sensitization training, whereas controls (n = 10) remained at baseline. Phase II: Two days after the decay of initial LTM, trained animals and matched controls were trained with two spaced tail shocks (TS). Tests at 2 and 24 h following training showed the induction of memory at 2 h (2 HR) and 24 h (24 HR) in previously trained animals, but not in matched controls. All T-SWR responses are normalized to baseline responding. In this and all subsequent figures, within-group significance is depicted by P-values within histograms, and between-group significance by the indicated comparisons; all probability values are two-tailed. Data are expressed as means ±SEM.
In Phase II, we assessed latent memory by retraining previously trained animals on the second consecutive day in which their mean T-SWR responses were below 120% baseline (which we operationally defined as forgetting, see Materials and Methods). We waited a second day until Phase II retraining to ensure that LTM for sensitization was forgotten (see Fig. 1). By the day of retraining, T-SWR responses had returned to pre-training levels in experimental animals (99 ± 4%, P > 0.5, Fig. 2, PRE). However, at this time point, the matched controls exhibited test responses that were modestly, but significantly, decremented (88 ± 5%) compared with their pre-training levels (P < 0.05, Fig. 2). The decremented response in controls may be due to nonspecific effects of prolonged maintenance in the behavioral testing chambers, as well as to partial habituation of the reflex induced by daily testing. Interestingly, this decrement was not observed in trained animals, even after daily testing throughout LTM expression and 4 d of forgetting (>1 wk, 101 ± 3%, NS, Fig. 4, below).
Figure 4.
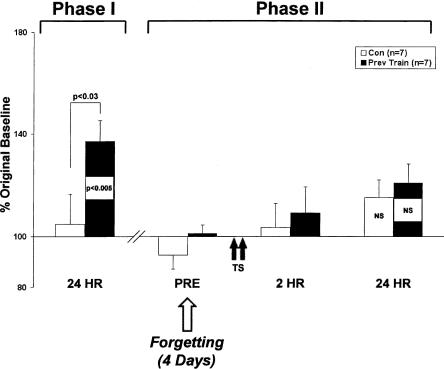
Latent memory decays by 4 d. Phase I: Trained animals (n = 7) exhibited robust LTM after sensitization training. Phase II: Four days following the decay of LTM, animals received two spaced tail shocks (TS). Memory was tested at 2 and 24 h following Phase II training. No memory was exhibited at 2 h or 24 h in previously trained animals or matched controls (n = 7).
In Phase II, both experimental and control animals were trained with two spaced tail shocks (ISI = 15 min, 15 mA, Fig. 2, Phase II). Phase II training led to the induction of three temporally distinct phases of memory (short- [10 min], intermediate- [2 h], and long-term [24 h]) in experimental animals. An overall analysis of variance revealed a significant difference among all the groups (F7,69 = 9.4, P < 0.001). Subsequent planned comparisons were then carried out (see Materials and Methods).
At the 2-h test, a within-group analysis revealed that previously trained animals exhibited significant 2-h memory (181 ± 22%, P < 0.005), and a between-group analysis showed that the experimental group differed significantly from controls (P < 0.01). At the 24-h test, a within-group comparison revealed that the experimental group exhibited significantly enhanced responding (122 ± 8%, P < 0.05), and a between-group comparison showed that the 24-h scores of the experimental animals were significantly elevated above controls (P < 0.02).
Sutton and colleagues (2002) had previously shown that two tail shocks induce memory lasting less than 30 min. Consistent with these previous observations, memory induced in control animals by Phase II training was similarly short-lived: Within-group comparisons showed that memory was absent at 2-h (105 ± 19%, NS) and 24-h (91 ± 7%, NS) tests (Fig. 2, Phase II).
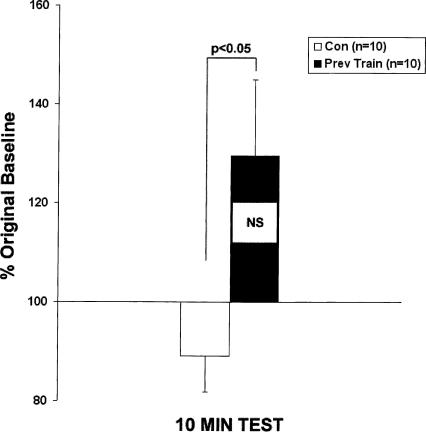
To explore the effects of retraining on short-term memory induction, we tested all animals 10 min following a single shock. While a single shock was sub-threshold for memory induction in controls (89 ± 7%, NS), short-term memory was induced in experimental animals (130 ± 16%); the experimental responses were significantly greater than controls (P < 0.05, Fig. 3). Thus, in contrast to the experimental animals, the Phase II training was insufficient to induce memory in control animals (see Discussion). Collectively, these data show that latent memory is induced in Aplysia by long-term sensitization training. This latent memory outlasts LTM expression by at least two days and is able to facilitate the induction of three temporally distinct phases of memory.
Figure 3.
Latent memory facilitates the induction of STM. In the same group of animals as in Figure 2, memory was examined 10 min following the first of two Phase II training shocks. A single tail shock produced significant enhancement of responses measured at 10 min in previously trained animals (n = 10) but not in matched controls (n = 10).
Latent memory decays by 4 d
Thus far, our data show that latent memory induced by sensitization training can be maintained for at least two days. How long does this latent memory last? To explore this question, we gave Phase I training, identical to that in the previous experiment (Fig. 2), to a group of animals (n = 7) with matched controls (n = 7), but waited until the fourth consecutive day of forgetting (LTM expression <120% baseline) to retrain with two shocks (Fig. 4). We then tested memory at 2 h and 24 h. An analysis of variance revealed no significant difference between experimental animals and matched controls when Phase II training was given after 4 d of forgetting (F7,48 = 1.97, P > 0.05). Experimental animals no longer exhibited enhanced responses at 2 h (109 ± 10%, NS) and 24 h (121 ± 7%, NS).
In a subset of animals (n = 4), we examined facilitation of short-term (10 min) memory induction with a single shock. Neither previously trained animals (115 ± 4%, NS) nor matched controls (107 ± 4%, NS) demonstrated significant memory at 10 min. Thus, under our training conditions, latent memory appears to decay by 4 d.
Latent memory can be induced even in the absenceof original memory
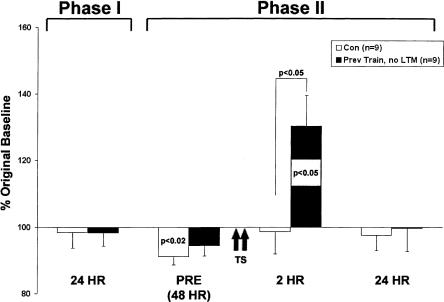
As described earlier, we developed a training protocol that optimized the forgetting of LTM, so that memory expression typically lasted 1–5 d (to facilitate the exploration of latent memory; see Materials and Methods). Under these conditions, some animals from each training session did not exhibit LTM after original sensitization training. These animals provided a unique opportunity to ask whether a long-lasting latent memory could be generated in the absence of original LTM expression. To explore this possibility, we retrained those animals (n = 9) that had shown no LTM for sensitization at 24 h (98 ± 4%, NS) and 48 h (94 ± 3%, NS) after training (Fig. 5, Phase I). When retrained with two spaced shocks 48 h after the original training, this group of animals nonetheless exhibited a form of latent memory. An analysis of variance demonstrated a significant overall difference between experimental animals and matched controls (F9,76 = 4.24, P < 0.001). Subsequent planned comparisons showed that experimental animals expressed a 2-h memory (130 ± 9%, P < 0.05, Fig. 5, Phase II) that was significantly greater than matched controls (P < 0.001). As in previous experiments (Figs. 2, 4), control animals did not exhibit significant 2-h memory following the two tail shocks (99 ± 7%, NS). In contrast to previous results (Fig. 2), however, we found that not all temporal phases of memory could be induced with retraining the experimental animals. Specifically, two tail shocks did not facilitate the induction of 24-h memory (100 ± 7%, NS, Fig. 5, Phase II). Finally, although the facilitation of short-term memory was not thoroughly explored, examination of a subset of these animals (n = 4) suggested that a 10-min memory is not induced with a single shock (126 ± 34%, NS).
Figure 5.
Latent memory can be induced in the absence of original LTM. Animals that did not express LTM after original training still exhibit the facilitated induction of memory at 2 h. Phase I: A subset of animals given long-term sensitization training (n = 9) did not express LTM following training. Phase II: Two days after the original training, two tail shocks induced significant 2-h memory, but not 24-h memory, in previously trained animals. Matched controls (n = 9) did not express 2-h or 24-h memory.
In a final experiment, with a group of animals which also did not exhibit overt LTM following Phase I training, we gave Phase II training 4 d after the original training (n = 7). Consistent with the results of retraining 48 h after original training (Fig. 5), in these animals we observed facilitation of 2-h memory (122 ± 9%, P < 0.05), but not 10-min (NS) or 24-h memory (NS).
These results suggest two main conclusions. First, latent memory can be induced by sensitization training even in the absence of overt memory resulting from that training. Second, this form of latent memory appears to be restricted, in that not all phases of memory were induced after retraining. Nonetheless, these data show that at least a reduced form of latent memory is not directly dependent on the induction of overt initial memory.
Discussion
Our results show that sensitization training of the T-SWR can induce a latent memory. Specifically, we find that the prior induction of LTM for sensitization can significantly enhance the subsequent induction of other memories, even after the original memory is apparently forgotten. Moreover, the original LTM training enhances subsequent memory induction in three mechanistically distinct temporal domains, STM, ITM, and LTM (Scholz and Byrne 1987; Castellucci et al. 1989; Goldsmith and Byrne 1993; Sutton et al. 2001, 2002). The latent memory induced by LTM training is maintained for at least two days after overt forgetting of the original experience but is gone by four days. Intriguingly, we also observed modest but significant latent memory even in the absence of any overt original LTM. Collectively, these data demonstrate the formation of latent memory within a reflex system well suited for the cellular and molecular analysis of this form of learning.
Latent memory in other systems
In the memory literature, latent memory is often referred to as “savings” (Ebbinghaus 1885; Nelson 1978). This phenomenon is widely observed in diverse animals (Matzel et al. 1992; Monk et al. 1996; Medina et al. 2001; Nicholson et al. 2003), including man (Ebbinghaus 1885; Nelson 1971, 1978; MacLeod 1988). Thus it is generally accepted in many systems that a memory can outlast its behavioral expression. A latent memory, however, can also develop in the absence of apparent original learning, a phenomenon referred to as latent learning. One of the earliest descriptions of latent learning was provided by Tolman and Honzik (1930), who examined maze exploration in rats. They found that rats that were initially unrewarded during maze exploration nonetheless showed facilitated learning when they were later taught to navigate the maze for a food reward. Although the retraining used to identify latent learning often involves the introduction of a motivationally relevant reward, this form of learning is reminiscent of the effect we see in a subset of our trained animals, which do not demonstrate original LTM for sensitization but do show the facilitated induction of a 2-h memory following retraining. These observations suggest the general conclusion that a training episode can have a clear impact on an animal even in the absence of a behavioral reflection of memory for that episode.
Given the ubiquitous nature of latent memory, surprisingly little is known about its underlying mechanisms. However, various forms of latent memory have been described in a number of preparations that are amenable to a mechanistic analysis. For example, in Aplysia latent memory was first demonstrated in a savings test in animals that had previously learned that a particular food was inedible (Susswein and Schwarz 1983). More recently, an interesting preliminary description of latent memory in the T-SWR was described (E.G. Antzoulatos, M.L. Wainwright, L.J. Cleary, and J.H. Byrne, pers. comm.). These authors report that 24 h after robust (4 d) sensitization training to one side of the body, although there is no apparent memory revealed by stimulation of the side contralateral to the original training, the subsequent induction of STM on the contralateral side is facilitated. This finding is consistent with our observations that latent memory can develop in the absence of overt initial expression of memory.
Two other studies of latent memory in molluscan systems have addressed possible mechanisms of savings. First, in Lymnaea, a recent report demonstrated that two bouts of an ITM induction protocol (separated by 24 h) can lead to the facilitated induction of LTM (Parvez et al. 2005). These authors also provided evidence that the mechanism for this form of savings may involve transcription and translation occurring in the cell body of a single identified neuron. Second, work in the marine snail Hermissenda has explored the properties of a single photoreceptor cell during savings for an associative (light-rotation) conditioning task (Matzel et al. 1992). The authors suggest that original training induces a Ca2+ hypersensitivity within the photoreceptor cell, which may be the principal contributor to savings seen within this system.
Although there are relatively few mechanistic studies of savings in mammalian systems, recent modeling predictions and experimental evidence in rabbits have suggested a possible savings mechanism during extinction training of eyelid conditioning (Medina et al. 2001). This preparation offers significant promise for the analysis of the mechanisms contributing to latent memory at a complex systems level (see Medina et al. 2002).
Mechanistic implications
What kinds of cellular mechanisms might underlie the forms of latent memory we have observed in the present study? Our data show that original training somehow alters the “state” of the CNS such that the response to subsequent training is enhanced. From this perspective, two principle questions emerge: First, what is the nature of the “state change” that underlies latent memory, and second, how does this state differentially modulate different temporal domains of memory?
In addressing the first question, using the T-SWR system, we now have the opportunity to ask informative questions about the cellular nature of the state change subserving latent memory. For example, one possible mechanism is that the impact of the tail shock is altered by previous training, so that the same tail shock has a greater effect in a subsequent retraining phase. To explore this possibility, following original training one could ask whether there is increased release of a modulatory neurotransmitter such as serotonin (5-HT), which is known to accompany the induction of sensitization memory, in response to tail shock (Levenson et al. 1999; Marinesco and Carew 2002). Sensitization training is also known to increase the response of tail sensory neurons to stimulation (Walters et al. 1983b). Thus, after overt behavioral forgetting, it is possible that the tail sensory neurons still encode some aspect of the original training as an increase in their responsiveness to stimuli. Finally, it is possible that the “molecular threshold” for the induction of subsequent memory may be modified by original training. If so, one might expect the underlying synaptic and molecular architecture for memory to be primed in some fashion. This could be assessed in a previously trained system by examining both synaptic plasticity and the signaling cascades known to contribute to the induction of sensitization memory in the T-SWR (Byrne et al. 1988; Sutton and Carew 2000; Sutton et al. 2001; Purcell et al. 2003; Sharma et al. 2003a, b; Sharma and Carew 2004; Sutton et al. 2004).
Turning to the second question, how might an altered state in the CNS modulate memory induction in different temporal domains? We know that the mechanistic requirements for memory are unique for STM, ITM, and LTM. STM involves the covalent modification of pre-existing proteins (for discussion see Kandel and Schwartz 1982), ITM requires the synthesis of new proteins, but not transcription (Sutton et al. 2001), and LTM requires both the synthesis of new gene products and new protein synthesis (Castellucci et al. 1989; Sutton et al. 2001). The two tail shocks we used in Phase II training do not normally lead to the induction of ITM or LTM (Sutton et al. 2002) and thus, at least in the naive state, would not be expected to induce the translation and transcription needed to carry memory into these extended temporal domains. However, our finding that latent memory can facilitate the induction of memory into the intermediate and long-term domains shows that these induction rules may not be the same in a system that has undergone a recent learning experience. This raises the interesting possibility that the 2-h and 24-h memories induced by retraining may not be mechanistically similar to those memories generated in a naive system. Thus, an informative next step will be to determine the molecular requirements of the savings memory in this system.
The above consideration raises the important question of whether all three mechanistically distinct forms of memory that we and others have identified in Aplysia are uniformly enhanced by a single latent memory, or whether they might be differentially enhanced. Our data suggest that the latter may be the case. We find that the facilitated induction of all three temporal phases of memory occurs only in animals that expressed overt LTM following the initial sensitization training. There is not comparable enhancement in those animals that did not express overt LTM after initial training; these animals exhibited only enhanced 2-h memory following Phase II training. Thus, the strength of the initial memory appears to determine the ability of subsequent training to facilitate the induction of different forms of memory. While these are, at this point, only hypothetical possibilities, our understanding of these temporal domains at behavioral, cellular, and molecular levels is quite extensive (Scholz and Byrne 1987, 1988; Sossin et al. 1994; Zhang et al. 1994; Sutton and Carew 2000; Sutton et al. 2001, 2002; Wainwright et al. 2002; Sharma et al. 2003a, b; Sharma and Carew 2004; Sutton et al. 2004; Wainwright et al. 2004). This understanding now provides a valuable platform from which we can examine alternative hypotheses in mechanistic detail.
In conclusion, we have established the existence, and some of the parametric features, of latent memory in the T-SWR of Aplysia. Since the induction and expression rules for different forms of memory are relatively well understood in this system, we are encouraged that it may now be possible to begin to understand the cellular and molecular architecture of a well-described feature of learning and memory that is common to virtually all systems.
Materials and Methods
Behavioral procedures
Adult Aplysia californica (weighing 150–250 g) were obtained commercially (Marinus and M-REP) and housed individually within a tank of circulating artificial seawater (ASW; Instant Ocean, Aquarium Systems) that was held at ∼16°C. Animals were fed dried seaweed three times a week.
At least four days before all experiments, animals were anesthetized (by cooling) and the parapodia surrounding the siphon were resected to better visualize the siphon during tail-elicited siphon withdrawal (T-SWR). T-SWR was tested by application of a water jet (0.5 s, 45 psi, Teledyne Water Pik) to a region ∼1 cm above the most posterior tip of the tail. Siphon withdrawal was measured as the time beginning immediately after application of the test stimulus (water jet) until the first signs of relaxation of the posterior portion of the siphon. We typically observed reflex responses lasting 3–8 sec.
In all experiments, the baseline reflex response was measured by the average of three test stimuli (ITI = 15 min). Animals with a single pre-test that varied by more than 20% of the mean were not used in the experiments. Less than 10% of all animals were excluded using this criterion. Twenty minutes after the last pre-test, animals received sensitization training consisting of electrical stimuli (2-s train of 10 ms, 15 mA DC pulses at 50 Hz) applied through a suction electrode to the tail midline, immediately below the convergence of the parapodia (Marinesco et al. 2004). This training site had sufficient spacing from the test site that it did not activate overlapping populations of SNs (Walters et al. 1983a; Sutton et al. 2004). Before all training trials, animals were moved into a secondary container, out of water, to access the shock site on the dorsal surface of the animal. With this protocol, we could reliably apply a fixed current without the shunting effects of application in seawater. After shock, animals were allowed to ink 20–30 sec before being replaced into their individual housing and testing units. Typically, animals were out of water a total of 30–40 sec for each shock application. In pilot experiments, we found that pulling animals out of water with no shock or mock stimulation had no significant effect on the T-SWR at 1.5 h or 24 h. After training, post-tests were given by application of water jet to the original test site. Post-tests were always taken by an observer blind to the training history of the animal. Control animals were tested in identical fashion with experimental animals at every time point.
Experimental protocol
To examine latent memory within the T-SWR of Aplysia, we first gave animals long-term sensitization training, which consisted of either four or five spaced shocks (2-s train of 10 ms, 15 mA DC pulses at 50 Hz, inter-shock interval [ISI] = 15 min, Sutton et al. 2002). While five shocks increased the percentage of animals showing LTM, the actual duration of LTM induced, on average, was not different between the training protocols of four versus five shocks. Moreover, the results obtained from animals originally trained with four shocks were not significantly different from those observed in animals originally trained with five shocks. Thus, data from these two groups were pooled in all analyses. LTM in trained animals typically lasted 1–5 d.
After training, LTM retention was assessed every 24 h with two T-SWR tests (ITI = 30 min) spaced to minimize any inter-trial effects. Control animals received identical tests throughout the experiment. To accurately describe the expression of sensitization memory (and subsequent forgetting), we established an expression threshold based upon experimental observations. In all experiments, average daily measurements equal to or above 120% pre-training levels (≥120%) were taken to reflect memory expression. We defined no memory, or forgetting, as average responses <120%.
All retraining occurred on the second or fourth consecutive day of forgetting (data in Figs. 2, 4, respectively). The actual duration of LTM varied among trained animals. Thus, each animal was retrained with a matched control which was treated identically but received no Phase I training. For retraining, two spaced shocks (2-s train of 10 ms, 15 mA DC pulses at 50 Hz) were applied to the original training site, and post-tests were taken at 2 and 24 h. In a subset of trained animals, we tested the effect of retraining on the induction of STM by testing the T-SWR 10 min after the first of two retraining shocks. This 10-min test had no effect on the subsequent 2-h and 24-h tests compared with the responses of those animals not tested at 10 min (data not shown).
Data analysis
Since the test scores were normally distributed, parametric statistics were used. An “outlier” rule was established: If a single score exceeded two standard deviations from the mean of the group containing that score, it was excluded from the analysis (a common winsorization rule; Dixon and Tukey 1968). In all analyses, this criterion was met in only six cases within 258 data points analyzed (<3% of all scores). An analysis of variance (ANOVA) was performed across all groups in Phase II (the ANOVA did not include Phase I data since the differences in this Phase resulted from experimental animals satisfying a response criterion [described above]). Subsequent planned comparisons were carried out using Student t-tests. Within-group comparisons were carried out examining difference scores between T-SW responses in the test condition compared with the average baseline response. Between-group comparisons were made using a t-test for independent means. All analyses were done with the Excel Analysis Pack (2003) and SPSS v10.0. All reported probabilities reflect two-tailed analyses.
Acknowledgments
We thank Dr. Michael Rugg for helpful initial discussions. We also thank our colleagues in Lab Carew for their helpful comments on an earlier draft of this manuscript. This work was supported by NIH grant R01 MH14-10183 to TJC.
Footnotes
Article and publication are at http://www.learnmem.org/cgi/doi/10.1101/lm.111506
References
- Byrne J.H., Eskin A., Scholz K.P. Neuronal mechanisms contributing to long-term sensitization in Aplysia. J. Physiol. (Paris) 1988;83:141–147. [PubMed] [Google Scholar]
- Castellucci V.F., Blumenfeld H., Goelet P., Kandel E.R. Inhibitor of protein synthesis blocks long-term behavioral sensitization in the isolated gill-withdrawal reflex of Aplysia. J. Neurobiol. 1989;20:1–9. doi: 10.1002/neu.480200102. [DOI] [PubMed] [Google Scholar]
- Cleary L.J., Lee W.L., Byrne J.H. Cellular correlates of long-term sensitization in Aplysia. J. Neurosci. 1998;18:5988–5998. doi: 10.1523/JNEUROSCI.18-15-05988.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon W.J., Tukey J.W. Approximate behavior of the distribution of winsorized t (trimming/winsorization 2). Technometrics. 1968;10:83–98. [Google Scholar]
- Ebbinghaus H.18851913. Memory: A contribution to experimental psychology (trans. H.A. Ruger and C.E. Bussenius). Columbia University, Teachers College; New York. [Google Scholar]
- Goldsmith J.R., Byrne J.H. Bag cell extract inhibits tail-siphon withdrawal reflex, suppresses long-term but not short-term sensitization, and attenuates sensory-to-motor neuron synapses in Aplysia. J. Neurosci. 1993;13:1688–1700. doi: 10.1523/JNEUROSCI.13-04-01688.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandel E.R., Schwartz J.H. Molecular biology of learning: Modulation of transmitter release. Science. 1982;218:433–443. doi: 10.1126/science.6289442. [DOI] [PubMed] [Google Scholar]
- Levenson J., Byrne J.H., Eskin A. Levels of serotonin in the hemolymph of Aplysia are modulated by light/dark cycles and sensitization training. J. Neurosci. 1999;19:8094–8103. doi: 10.1523/JNEUROSCI.19-18-08094.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubow R.E., Gewirtz J.C. Latent inhibition in humans: Data, theory, and implications for schizophrenia. Psychol. Bull. 1995;117:87–103. doi: 10.1037/0033-2909.117.1.87. [DOI] [PubMed] [Google Scholar]
- Lubow R.E., Moore A.U. Latent inhibition: The effect of nonreinforced pre-exposure to the conditional stimulus. J. Comp. Physiol. Psychol. 1959;52:415–419. doi: 10.1037/h0046700. [DOI] [PubMed] [Google Scholar]
- MacLeod C.M. Forgotten but not gone: Savings for pictures and words in long-term memory. J. Exp. Psychol. Learn. Mem. Cogn. 1988;14:195–212. doi: 10.1037//0278-7393.14.2.195. [DOI] [PubMed] [Google Scholar]
- Marinesco S., Carew T.J. Serotonin release evoked by tail nerve stimulation in the CNS of aplysia: Characterization and relationship to heterosynaptic plasticity. J. Neurosci. 2002;22:2299–2312. doi: 10.1523/JNEUROSCI.22-06-02299.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinesco S., Wickremasinghe N., Kolkman K.E., Carew T.J. Serotonergic modulation in aplysia. II. Cellular and behavioral consequences of increased serotonergic tone. J. Neurophysiol. 2004;92:2487–2496. doi: 10.1152/jn.00210.2004. [DOI] [PubMed] [Google Scholar]
- Matzel L.D., Collin C., Alkon D.L. Biophysical and behavioral correlates of memory storage, degradation, and reactivation. Behav. Neurosci. 1992;106:954–963. doi: 10.1037//0735-7044.106.6.954. [DOI] [PubMed] [Google Scholar]
- Medina J.F., Garcia K.S., Mauk M.D. A mechanism for savings in the cerebellum. J. Neurosci. 2001;21:4081–4089. doi: 10.1523/JNEUROSCI.21-11-04081.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina J.F., Christopher Repa J., Mauk M.D., LeDoux J.E. Parallels between cerebellum- and amygdala-dependent conditioning. Nat. Rev. Neurosci. 2002;3:122–131. doi: 10.1038/nrn728. [DOI] [PubMed] [Google Scholar]
- Monk C.M., Gunderson V.M., Grant K.S., Mechling J.L. A demonstration of the memory savings effect in infant monkeys. Dev. Psychol. 1996;32:1051–1055. [Google Scholar]
- Nelson T.O. Savings and forgetting from long-term memory. Journal of Verbal Learning and Verbal Behavior. 1971;10:568–576. [Google Scholar]
- Nelson T.O. Detecting small amounts of information in memory: Savings for nonrecognized items. J. Exp. Psychol. [Hum Learn] 1978;4:453–468. [Google Scholar]
- Nicholson D.A., Sweet J.A., Freeman Jr. , J.H. Long-term retention of the classically conditioned eyeblink response in rats. Behav. Neurosci. 2003;117:871–875. doi: 10.1037/0735-7044.117.4.871. [DOI] [PubMed] [Google Scholar]
- Parvez K., Stewart O., Sangha S., Lukowiak K. Boosting intermediate-term into long-term memory. J. Exp. Biol. 2005;208:1525–1536. doi: 10.1242/jeb.01545. [DOI] [PubMed] [Google Scholar]
- Plotkin H.C., Oakley D.A. Backward conditioning in the rabbit (Oryctolagus cuniculus). J. Comp. Physiol. Psychol. 1975;88:586–590. doi: 10.1037/h0076426. [DOI] [PubMed] [Google Scholar]
- Purcell A.L., Sharma S.K., Bagnall M.W., Sutton M.A., Carew T.J. Activation of a tyrosine kinase-MAPK cascade enhances the induction of long-term synaptic facilitation and long-term memory in Aplysia. Neuron. 2003;37:473–484. doi: 10.1016/s0896-6273(03)00030-8. [DOI] [PubMed] [Google Scholar]
- Scholz K.P., Byrne J.H. Long-term sensitization in Aplysia: Biophysical correlates in tail sensory neurons. Science. 1987;235:685–687. doi: 10.1126/science.2433766. [DOI] [PubMed] [Google Scholar]
- Scholz K.P., Byrne J.H. Intracellular injection of cAMP induces a long-term reduction of neuronal K+ currents. Science. 1988;240:1664–1666. doi: 10.1126/science.2837826. [DOI] [PubMed] [Google Scholar]
- Sharma S.K., Carew T.J. The roles of MAPK cascades in synaptic plasticity and memory in Aplysia: Facilitatory effects and inhibitory constraints. Learn. Mem. 2004;11:373–378. doi: 10.1101/lm.81104. [DOI] [PubMed] [Google Scholar]
- Sharma S.K., Bagnall M.W., Sutton M.A., Carew T.J. Inhibition of calcineurin facilitates the induction of memory for sensitization in Aplysia: Requirement of mitogen-activated protein kinase. Proc. Natl. Acad. Sci. 2003a;100:4861–4866. doi: 10.1073/pnas.0830994100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma S.K., Sherff C.M., Shobe J., Bagnall M.W., Sutton M.A., Carew T.J. Differential role of mitogen-activated protein kinase in three distinct phases of memory for sensitization in Aplysia. J. Neurosci. 2003b;23:3899–3907. doi: 10.1523/JNEUROSCI.23-09-03899.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sossin W.S., Sacktor T.C., Schwartz J.H. Persistent activation of protein kinase C during the development of long-term facilitation in Aplysia. Learn. Mem. 1994;1:189–202. [PubMed] [Google Scholar]
- Susswein A.J., Schwarz M. A learned change of response to inedible food in Aplysia. Behav. Neural Biol. 1983;39:1–6. doi: 10.1016/s0163-1047(83)90535-6. [DOI] [PubMed] [Google Scholar]
- Sutton M.A., Carew T.J. Parallel molecular pathways mediate expression of distinct forms of intermediate-term facilitation at tail sensory-motor synapses in Aplysia. Neuron. 2000;26:219–231. doi: 10.1016/s0896-6273(00)81152-6. [DOI] [PubMed] [Google Scholar]
- Sutton M.A., Masters S.E., Bagnall M.W., Carew T.J. Molecular mechanisms underlying a unique intermediate phase of memory in aplysia. Neuron. 2001;31:143–154. doi: 10.1016/s0896-6273(01)00342-7. [DOI] [PubMed] [Google Scholar]
- Sutton M.A., Ide J., Masters S.E., Carew T.J. Interaction between amount and pattern of training in the induction of intermediate- and long-term memory for sensitization in aplysia. Learn. Mem. 2002;9:29–40. doi: 10.1101/lm.44802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton M.A., Bagnall M.W., Sharma S.K., Shobe J., Carew T.J. Intermediate-term memory for site-specific sensitization in aplysia is maintained by persistent activation of protein kinase C. J. Neurosci. 2004;24:3600–3609. doi: 10.1523/JNEUROSCI.1134-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolman E.C., Honzik C.H. Introduction and removal of reward, and maze performance in rats. University of California Publications in Psychology. 1930;4:257–275. [Google Scholar]
- Wainwright M.L., Zhang H., Byrne J.H., Cleary L.J. Localized neuronal outgrowth induced by long-term sensitization training in aplysia. J. Neurosci. 2002;22:4132–4141. doi: 10.1523/JNEUROSCI.22-10-04132.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wainwright M.L., Byrne J.H., Cleary L.J. Dissociation of morphological and physiological changes associated with long-term memory in aplysia. J. Neurophysiol. 2004;92:2628–2632. doi: 10.1152/jn.00335.2004. [DOI] [PubMed] [Google Scholar]
- Walters E.T., Byrne J.H., Carew T.J., Kandel E.R. Mechanoafferent neurons innervating tail of Aplysia. I. Response properties and synaptic connections. J. Neurophysiol. 1983a;50:1522–1542. doi: 10.1152/jn.1983.50.6.1522. [DOI] [PubMed] [Google Scholar]
- Walters E.T., Byrne J.H., Carew T.J., Kandel E.R. Mechanoafferent neurons innervating tail of Aplysia. II. Modulation by sensitizing stimulation. J. Neurophysiol. 1983b;50:1543–1559. doi: 10.1152/jn.1983.50.6.1543. [DOI] [PubMed] [Google Scholar]
- Zhang F., Goldsmith J.R., Byrne J.H. Neural analogue of long-term sensitization training produces long-term (24 and 48 h) facilitation of the sensory-to-motor neuron connection in Aplysia. J. Neurophysiol. 1994;72:778–784. doi: 10.1152/jn.1994.72.2.778. [DOI] [PubMed] [Google Scholar]