Abstract
Objective:
To prospectively evaluate the accuracy of frozen sectioning (FS) of the pancreatic transection margin and its influence on surgery during resection of intraductal papillary and mucinous neoplasms (IPMNs).
Summary Background Data:
Preoperative assessment of IPMN extension is difficult and transection margin is frequently tumoral on the surgical specimen.
Patients and Methods:
FS was performed in 127 patients who underwent partial pancreatectomy for IPMN from 1996 to 2004, corresponding to 90 pancreaticoduodenectomies (1–4 successive FS; total = 132), 25 distal pancreatectomies (1–2 FS; total = 27), and 12 medial pancreatectomies (2–4 FS; total = 29). Dysplasia was graded in both main (MD) and branch ducts (BD), and pancreatectomy was extended if FS revealed at least IPMN adenoma on the MD or borderline IPMN on BD (defined as “significant” lesions).
Results:
The 188 FS revealed that MD and BD epithelium comprised significant noninvasive lesions in 49 and 13 cases, respectively, and infiltrating carcinoma in 4 other ones. Definitive examination corroborated FS in 176 of 188 cases (94%). Altogether, 54 of 188 (29%) FS comprised significant lesions that resulted in 46 additional resections in 38 patients (30%). Eight patients did not have additional resection because of either high operative risk or preoperative diagnosis of noncurable infiltrating carcinoma. The 134 FS without significant lesions were associated with 7 additional resections mainly because of macroscopic suspicion of another tumor location. Conflicting results between FS and definitive examination resulted in inadequate extent of pancreatectomy in 4 patients (3%).
Conclusions:
Results of FS of the transection margin are confirmed by definitive examination in 94% of cases. According to our protocol, FS changes the extent of resection in 30% of patients and allows adequate resection in 97% of patients.
Frozen sectioning (FS) of the transection margin during pancreatectomy for intraductal papillary mucinous neoplasms (IPMNs) was prospectively evaluated in 127 patients. Pancreatectomy was extended if FS revealed at least IPMN adenoma on the main duct, or borderline IPMN on branch duct. FS resulted in additional resection in 30% of patients. Definitive examination corroborated FS in 94% of cases.
Intraductal papillary mucinous neoplasms (IPMNs) of the pancreas are rare exocrine tumors, which have been recently defined and classified by the WHO.1 They include a spectrum of dysplasia ranging from minimal mucinous hyperplasia to invasive carcinoma. IPMNs are extensive tumors that often spread along the ductal tree.1–3 They can involve the main and/or the branch ducts, and the risk of malignant transformation is lower for tumor localized in the latter.4–6
Surgical resection allows to eradicate these precancerous or invasive lesions and should be tailored to the tumor topography, to perform a resection as complete as possible with minimal risk of endocrine and exocrine insufficiency. However, preoperative imaging is not reliable for the precise evaluation of tumor extension7,8 and preoperative biopsies often underestimate tumor grade.9 Our team previously demonstrated in a limited series that frozen sectioning (FS) of the pancreatic resection margin, studying only presence or absence of IPMN, was better than preoperative morphologic assessment to accurately adapt the extent of pancreatectomy.7 We then prompted to prospectively analyze: 1) the value of FS of the pancreatic resection margin assessing both the respective involvement of the main pancreatic duct and its sub-branches and the degree of dysplasia; and 2) the influence of FS on the surgical procedure.
PATIENTS AND METHODS
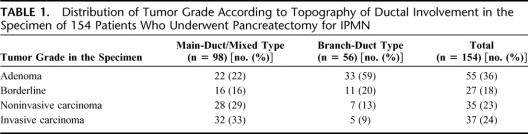
Between January 1996 and September 2004, 154 consecutive patients underwent pancreatic resection for IPMN at our institution. There were 75 men and 79 women, with a median age of 63 years (range, 29–81 years). Preoperative assessment routinely included abdominal CT scan, endoscopic ultrasonography, and either endoscopic retrograde pancreatography (ERCP) or magnetic resonance cholangiopancreatography. The diagnosis of IPMN was confirmed by histologic examination of the pancreatic resected specimen according to criteria of the WHO.1,2 There were 56 branch duct variants, 13 main duct, and 85 mixed variants. Distribution of tumor grade according to topography of ductal involvement in the specimen is summarized in Table 1.
TABLE 1. Distribution of Tumor Grade According to Topography of Ductal Involvement in the Specimen of 154 Patients Who Underwent Pancreatectomy for IPMN
FS of the pancreatic resection margin was routinely used with the exception of one-step total pancreatectomy (n = 5), completion pancreatectomy after previous resection (n = 5), enucleation for small peripheral unilocular branch duct localization (n = 6), impossibility to perform a larger resection than that planned (n = 5), or diagnosis of IPMN not established preoperatively (n = 6). FS of the pancreatic transection margin was performed in 127 pancreatectomies defined according to the planned resection, including 90 pancreaticoduodenectomies, 25 distal pancreatectomies, and 12 medial pancreatectomies. The planned resection was chosen according to the whole preoperative imaging assessment, to remove the pancreatic area affected by IPMN lesions and to preserve the parenchyma with no dilated ducts or possibly involved by passive dilatation. Of the 90 pancreaticoduodenectomies, 1 (n = 57), 2 (n = 26), 3 (n = 5), to 4 (n = 2) successive FSs were performed, giving a total number of 132 FSs. Of the 25 distal pancreatectomies, 1 (n = 23) to 2 (n = 2) FSs were performed, giving a total number of 27 FSs. Of the 12 medial pancreatectomies, 2 (right and left; n = 8), 3 (n = 3), to 4 (n = 1) FSs were performed, giving a total number of 29 FSs.
FS was performed either on the resected specimen or on a fresh slice of pancreatic cut surface harvested immediately after transection. All pancreatic transections were performed with a scalpel, as often as possible on a pancreatic segment without branch duct dilatation. When necessary, guide mark stitches were placed by the surgeon to orient the resected specimen. One 5-μm section was cut in a cryostat at −20°C, dried and colored by hematoxylin and eosin, mounted, and analyzed under light microscopy. In case of eroded epithelium, several seriated sections were analyzed. The result of FS included description of the main duct and branch ducts epithelium (normal, IPMN adenoma, borderline IPMN, IPMN with carcinoma in situ). Moreover, foci of invasive carcinoma were noted. Because of the frequent coexistence of various degrees of epithelial atypia, lesions were categorized in each type of duct (ie, main duct and branch ducts) according to the most severe degree of dysplasia observed. The FS result was transmitted by phone to the senior surgeon and written both in the pathologic report and in the operative report. The FS fragment was systematically fixed in formalin 10%, embedded in paraffin, and analyzed for definitive histology. The results of FS of the pancreatic cut surface were compared with the results of definitive examination.
Pancreatectomy was extended if FS revealed: 1) at least IPMN adenoma on the main duct; b) at least borderline IPMN on branch ducts; or c) invasive carcinoma. We denoted these lesions as “significant” ones. Extension of pancreatectomy depended on the first resection performed. After pancreaticoduodenectomy, a 2- to 3-cm length additional segment was resected with preservation of the splenic vessels. After left pancreatectomy, additional resection either included the head-neck junction on the left side of the common bile duct or was performed by means of completion pancreatectomy. After medial pancreatectomy, an additional resection was performed on the side containing “significant” lesions according to the techniques described above. A second FS was performed on the additional specimen with the same protocol. If needed, a third or even a fourth additional pancreatic resection was performed. Additional FS was not performed in case of completion pancreatectomy or when an additional resection was not indicated because of technical difficulties or general condition of the patient.
Statistical comparisons were done with the χ2 test. Significance was accepted for a P value <0.05.
RESULTS
Results of FS
The 188 FSs revealed that: 1) main duct epithelium was normal (n = 120, 65%), eroded (n = 15, 8%), or comprised IPMN adenoma (n = 33, 18%), borderline IPMN (n = 11, 6%), or noninvasive carcinoma (n = 5, 3%) lesions; 2) branch duct epithelium was normal (n = 106, 58%), or comprised IPMN adenoma (n = 65, 35%), borderline IPMN (n = 8, 4%), or noninvasive carcinoma (n = 5, 3%) lesions. Furthermore, FS revealed infiltrating carcinoma in 4 cases corresponding to main duct IPMN in 3 cases and to branch duct type in 1 case. No FS revealed eroded epithelium on branch ducts. Of the 188 FSs, 84 (45%) were normal.
Altogether, 54 of 188 (29%) FSs comprised significant lesions, observed in 38 patients (30%). The first analyzed FS comprised significant lesions in 25 of 90 (28%) pancreaticoduodenectomies, in 7 of 25 (28%) left pancreatectomies, and in 6 of 12 (50%) medial pancreatectomies (corresponding to 7 FSs in 6 patients, including 5 with significant lesions at FS on the right side of the specimen and one with significant lesions on both sides) (not significant = NS). At the second FS analysis, lesions were considered as significant as well in 8 of 38 patients (21%).
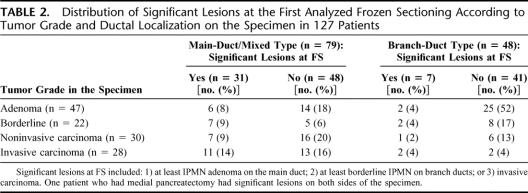
Thirteen of the 28 (46%) IPMNs with invasive carcinoma versus 25 of the 99 (25%) noninvasive IPMNs had significant lesions on the first analyzed FS (χ2 = 4.668; P = 0.03). Thirty-one of the 79 (39%) main duct or mixed-type IPMNs versus 7 of the 48 (15%) branch duct-type IPMNs had significant lesions on the first analyzed FS (χ2 = 8.657; P < 0.01) (Table 2).
TABLE 2. Distribution of Significant Lesions at the First Analyzed Frozen Sectioning According to Tumor Grade and Ductal Localization on the Specimen in 127 Patients
Comparison of FS: Definitive Pathologic Examination
Definitive examination corroborated FS in 176 of 188 cases (94%). The 12 conflicting results included 9 cases of “underestimation” by FS (normal epithelium versus IPMN adenoma [in main duct: n = 1; in branch ducts: n = 5]; normal epithelium versus borderline IPMN or noninvasive carcinoma [in main duct: n = 2]; borderline IPMN versus invasive carcinoma [in main duct: n = 1]) and 3 cases of “overestimation” by FS (IPMN adenoma versus normal epithelium [in branch ducts: n = 1]; borderline IPMN versus IPMN adenoma [in main duct: n = 1); infiltrative carcinoma versus chronic pancreatitis [n = 1]). Conflicting results were observed in 13% (8 of 64) of the FSs performed from January 1996 to December 2000 and in 6% (4 of 63) of the FSs performed from January 2001 to September 2004 (NS).
Influence of FS on Surgery
The 54 FSs with “significant” lesions resulted in 46 additional resections. Indeed, 8 patients with significant lesions did not have additional resection due to either high operative risk (n = 3), preoperative diagnosis of infiltrative carcinoma with incomplete resection along the celiac trunk (n = 1), or positive retroperitoneal lymph nodes (n = 1), misinterpretation of FS result (corresponding to IPMN adenoma on main duct epithelium, n = 2), or absence of preoperative information about a possible total pancreatectomy (n = 1). The 46 additional resections were performed in 38 patients (30%). In these 38 patients who had subsequent resection because of “significant” lesions at the first FS, we classified the lesions observed in the whole second resection specimen: these lesions were less severe in 13 (34%), identical in 17 (45%), and more severe to those observed at the first FS in 6 (16%). In 2 patients (5%), analysis of the second resection specimen did not reveal any aspect of IPMN despite extensive sampling.
The 134 FSs without “significant” lesions were associated with additional resection in 7 patients due to either macroscopic suspicion of tumor in the adjacent pancreatic segment (n = 5, including 3 cases with eroded main duct epithelium), mild dysplasia IPMN at the junction branch-main duct (n = 1), or misinterpretation of FS result by the surgeon (corresponding to IPMN adenoma on branch ducts, n = 1). Of these 7 patients, 6 had an additional partial left resection following pancreaticoduodenectomy.
Of the 12 conflicting results between FS and definitive examination, 4 (3%) resulted in inadequate extension of pancreatectomy, insufficient in 3 cases (normal epithelium versus IPMN adenoma in main duct: n = 1; normal epithelium versus borderline IPMN or noninvasive carcinoma in main duct: n = 2) and excessive in 1 case (infiltrative carcinoma versus chronic pancreatitis: n = 1). The latter one was the only one that resulted in inadequate extension of pancreatectomy since January 2001.
Altogether, of the 127 patients in whom a partial pancreatectomy with FS was planned, 9 patients (7%) ultimately underwent total pancreatectomy.
DISCUSSION
This work prospectively evaluated, in a large unicentric series of patients, both the value and influence of FS of pancreatic resection margin in the surgical management of IPMN. We demonstrated that FS is useful since it changed the extent of resection in 30% of patients and reliable since it allowed adequate resection in 97% of patients according to our protocol. This prospective study was initiated after our team demonstrated that FS of the pancreatic resection margin was better than preoperative morphologic assessment to accurately adapt extent of pancreatectomy.7 Several studies have demonstrated that imaging procedures, including abdominal CT scan, endoscopic ultrasonography, ERCP, or magnetic resonance magnetic resonance cholangiopancreatography, are not reliable to predict the extent of IPMN lesions.8,10–13 Indeed, pancreatic resection margins frequently comprise residual IPMN.12,14,15 Conversely, all pancreatic segments, including ductal dilatation, should not be resected because dilatation can be due only to obstruction by mucus either upstream or downstream from the tumor location. We then developed a protocol of FS of the pancreatic resection margin that precisely studied both dysplasia grading (according to the WHO classification) and ductal localization (ie, main duct and branch ducts). This was permitted by analysis of a slice sampled from the transection parenchyma with a scalpel for minimal tissue damage. We defined as “significant” lesions requiring additional resections: 1) at least IPMN adenoma on the main duct; 2) at least borderline IPMN on branch ducts; or 3) invasive carcinoma.
The arguments that prompted us to select this definition of significant lesions were the high rate of invasive carcinoma in patients with IPMN involving the main duct, ranging from 45% to 60%.5,6,13,14,16,17 Then, even a minimal lesion involving the main duct was treated by additional resection. Conversely, we tolerated residual mild dysplasia limited to branch ducts for the following reasons: 1) the rate of invasive lesions is very low in branch ducts IPMN, ranging from 6% to 12%5,6,13,14,16,17; 2) the “natural history” of branch ducts IPMN is slow according to some studies, which reported few morphologic changes without symptoms with a mean follow-up ranging from 30 to 55 months.18,19 So, it can be hypothesized that branch duct IPMN adenoma that is left in place carries a very delayed risk of recurrent evolutive disease4,5,20 with a long-term survival equivalent to that observed after more extensive pancreatectomies, especially if performed for invasive tumor; 3) without ductal dilatation, branch duct IPMN adenoma lesions cannot be distinguished from hyperplastic reactive epithelial changes that are frequent, secondary to obstruction or chronic pancreatitis.2,17,21 So, resection of all epithelial abnormalities can lead to excessive extent of pancreatectomy; and 4) the rate of total pancreatectomies in surgical series of IPMN can rise up to 23%.21 Since the metabolic consequences of total pancreatectomies are often deleterious, preservation of pancreatic parenchyma is a reasonable goal in case of benign lesions, particularly in old or high-risk patients.17,22
In the present study, the definition of “cutoff” lesion is very strict and precise as compared with previously reported data. In our early experience, we did not report separately branch ducts and main duct involvement.7 The Verona group considered as positive only high-grade dysplastic alteration of the ductal epithelium, without specifying the topography of ductal involvement.23 More recently, this group in association with that of the Massachusetts General Hospital in Boston reported their experience concerning patients with main duct IPMN and integrated low-grade dysplasia as an “equivocal” result, and moderate- and high-grade dysplasia as “positive” results.13 Our study highlights the importance to analyze also the branch ducts at the surgical margin since we observed 6 cases of FS with carcinoma in such location. In a last study, no clear cutoff value was defined.8 To our knowledge, no other work reported the routine use of FS in the surgical management of IPMN.
In the present study, analyses of the surgical margin obtained at FS and after fixation, both noticed in the same pathologic report, were identical in 94% of cases. Accuracy of FS improved with time with 2 times less conflicting results in the most recent half of patients. Conflicting results between FS and definitive examination resulted in inadequate extent of pancreatectomy according to our protocol in only 3% of patients. Indeed, conflicting results in the same group of either significant or nonsignificant results would not have modified extent of pancreatectomy. At FS, we interestingly found an eroded main duct epithelium in 8% of cases, a result comparable to those of Falconi et al.23 In our series, we performed an additional resection in some patients with eroded epithelium because this finding was associated to macroscopic suspicion of persisting ductal tumor. Moreover, considering that an eroded epithelium is clearly associated with recurrence in the pancreatic remnant in the series of Falconi et al,23 we think that presence of eroded epithelium on the main duct should routinely lead to an additional resection.
Altogether, 29% of FSs comprised significant lesions, which were observed in 30% of patients. At the first FS analyzed, the rate of significant lesions was greater in case of main duct or mixed-type IPMN (39% versus 15% in case of branch duct involvement, P < 0.01), as well as in case of invasive IPMN on the resected specimen (46% versus 25% in case of noninvasive IPMN, P = 0.03). Although predominantly useful in these subgroups of patients, FS can reveal significant lesions at the surgical margin in all settings of this disease. The rate of significant lesions on the first analyzed FS was almost the same if the planned resection was a pancreaticoduodenectomy or a left pancreatectomy (28%) but rose to 50% in case of planned medial pancreatectomy. Concerning this latter resection, FS revealed significant lesions on the right side of the specimen in half of patients; this result confirms that indications of medial pancreatectomy for IPMN are difficult to define24 and should be balanced to that of pancreaticoduodenectomy extended to the neck.
FS resulted in extension of the planned resection in 30% of patients. Of the patients who had additional resection because of significant lesions at the first FS, 95% had IPMN lesions on the second resection specimen and 21% presented significant lesions on the second FS as well. These results underline that FS allows to guide the extent of pancreatectomy in this disease.
In the present series, 6 patients had 3 successive FS and 2 had 4 to preserve as much parenchyma as possible. This policy resulted in a low rate (7%) of total pancreatectomy, as compared with the 10% to 23% rates reported in other series.12,14,15,21,23,25,26 In our study, 8 patients with significant lesions did not have additional resection. FS of the pancreatic margin was not clearly indicated in some of these patients who were at high operative risk for an extended resection or presented a noncurable invasive carcinoma diagnosed preoperatively. However, in high-risk patients, FS can help to choose the optimal surgical strategy according to the preoperative findings: as an example, leaving IPMN adenoma in main duct is acceptable in a high-risk patient while a proven carcinoma could lead to accept an additional resection. In 1 of our patients, absence of preoperative information about a possible total pancreatectomy impeded to perform an adequate resection. This underlines the need to inform all patients about the procedure and the possibility to extend the resection up to a total pancreatectomy. We observed 3 cases of misinterpretation of FS results between the pathologist and the surgeon. That points out the need of a reliable communication during transmission of FS results, which should be discussed with the surgeon in difficult cases. The decision of performing an additional resection should be taken on a dialogue basis.
The theoretical limit of using FS to adapt extent of resection is the existence of discontinuous IPMN lesions, which account for 6% of specimens in 2 surgical series.27,28 This 6% rate is close to the 7% to 8% rates of recurrence in the pancreatic remnant reported after partial pancreatectomy.13,14,21 Some of these recurrences occurred after partial pancreatectomy with normal transection margin.13,14,21 When they involve the main duct, detection of discontinuous lesions could rely on preoperative wirsungoscopy with staged biopsies. However, this technique is only feasible when the main duct is dilated and has only been reported by a few groups.8,29
CONCLUSION
During pancreatectomy for IPMN, FS of the pancreatic margin is useful. Our results demonstrate that both main duct and branch ducts should be analyzed during this procedure. Indeed, “positive” margins are more frequent in patients with main duct or mixed variants but can be also observed in patients with branch duct IPMN. The use of the WHO classification to describe the frozen section allows reliable results. Our approach, which aimed to remove all neoplastic epithelium on main duct but tolerated persistent IPMN adenoma on branch ducts, needs to be validated by follow-up comparing patients with normal transection margin to those with mild dysplasia in branch ducts. Comparative evaluation of both survival and risk of recurrence will probably need several years, considering the slow natural history of branch ducts IPMN.
Discussions
Dr. Di Carlo: I thank Pofessor Gouma for giving me the opportunity to comment on this paper.
Intraductal papillary mucinous tumors are to be considered no more “a rare” neoplasm since they now account for 20% to 30% of pancreatic resections performed for tumors. Dr. Sauvanet and his colleagues should be congratulated for their excellent presentation.
Your casuistic is impressive with more than 150 resected IPMTs in less than 9 years. Facing this tumor, you suggest to always perform a frozen section examination of the transection margin, and I think that all of us agree with this statement. The reliability of the frozen sections examination in your hands is high (94%), and it allowed to change the extent of the resection in about 30% of patients. In 8 patients, the resection was not extended despite the finding of a significant lesion of the cut margin. I agree with this attitude because the aim to achieve a negative resection margin must be a result of patient-tailored cost/benefit balance. However, some aspects deserve further comments.
First, the group of Verona suggested that the presence of the loss of epithelium, that they call denudation, on the cut surface has to be considered a positive margin because of the finding of local recurrence in the follow-up. You found in fact 15 patients with eroded epithelium in the transection margin, and you performed an additional resection only in some of them. The question is: do you think to include in the near future the ductal epithelial denudation or erosion within the “significant” lesions that need important pancreatic resection?
Second, you stated that, in a high-risk patient, leaving IPMT adenoma of the main duct is acceptable. But what is your policy in the “normal” patient? Do you think that the findings of main-duct adenoma on the transection margin justify a total pancreatectomy?
Third, the originality of your work relies mostly on the evaluation either of the main duct and of branch ducts on the transection margin. You suggest not to extend the resection when adenoma are found on the branch duct but to extend the pancreatectomy when adenoma is found on the main duct. This is based on the assumption that the branch-type IPMT has less malignant potential than the main duct type. However, the natural history of this tumor is far to be completely clarified.
Have you some data about the follow-up of the patients with branch-type adenoma on the transection marging?
Thank you very much for the attention.
Dr. Sauvanet: Thank you very much for your kind comments. My answer to your question is that I agree with the proposition of the Verona group. The publication of the Verona group was in 2001, so, when we began our study in 1996, we were not aware of this problem. We actually had some patients with eroded epithelium, only on main duct. Our pathologists confirmed that the epithelium was eroded, but we performed an additional resection if the transection margin was macroscopically suspect or when the surgeon was anxious. In some cases, we did not extend the resection. Clearly, in the experience of the Verona group, eroded epithelium was associated with a high risk of recurrence, so an additional resection should be recommended. Presently, it is our attitude.
Your second question is what to do in a low-risk patient when frozen section reveals adenoma on the main duct. The first step of the answer is to inform preoperatively all patients in whom a total pancreatectomy can be performed with an acceptable risk. In our series, some patients had IPMN lesions much more extended in the pancreas than suggested by preoperative imaging, so a limited pancreatectomy was not able to remove all significant lesions. So we now systematically inform of a possible total pancreatectomy all the patients who can sustain it. In a low-risk patient with a long life expectancy who agrees with a possible total pancreatectomy, we extend the resection up to a total pancreatectomy if FS reveals adenoma on the main duct, but this attitude is debatable.
Your last question is about the patient in whom branch duct adenoma is left in place. This finding is encountered in 35% of the patients in this series; that is important. The only possibility to determine what will be the effect of this feature is to follow the patients for a very long period. I have, at present, not the answer to your question. In our institution, the team of gastroenterologists is not in favor of routine surgery in branch duct IPMN, so approximately 40% of patients with branch duct IPMN are not operated and are followed by periodic imaging. Recently, the gastroenterologists in our institution estimated that the actuarial risk of both invasive and noninvasive carcinoma in patients with branch duct IPMN was 15% at 5 years. This result was established with the retrospective study of the preoperative course of the patients we operated and the prospective follow-up of patients with macroscopic branch duct lesions who were not operated. In the patients of our series in whom we left in place mainly microscopic lesions, I presume that the risk of distant malignancy will be lower. So, in patients in whom we left branch duct adenoma, we will need an at least 5 years but more probably 10 years follow-up to determine their exact future.
Dr. Cameron: Mr. Chairman, I want to make a few comments because this is an excellent series, certainly one of the largest series of IPMTs from one institution that I am aware of. The first comment is: where are they coming from? Our last report was on 150 patients with IMPTs, and we have subsequently operated on another 60 or 70 patients, and like your series, ours are virtually all from the last 10 years. Twenty years ago, we had ERCPs and CT scans, and we were not identifying these patients. We think there is an increased prevalence of them, and I wonder what your thoughts are.
Secondly, on frozen section, the only difficulty we have had is when we have confused an IPMN for a PANIN, a pancreatic intraepithelial neoplasm, 2 precursors for adenocarcinoma. For a PANIN, I think most people would not recommend increasing the resection; but if it were an IPMN, you would most likely increase it. So we have been confused and fooled a couple of times, and I wonder whether you have had the same experience.
The third is a similar question to Prof. Di Carlo's: would you chase an adenoma at the margin and do a further resection, for a patient you did a Whipple on with a main duct IPMN adenocarcinoma? Would you chase an adenoma in the main duct all the way to the end and do a total pancreatectomy?
My last question is: branch duct IPMNs do have a lower incidence of invasive adenocarcinoma than main duct IPMNs, but not by much. We have an incidence of 40% cancer in overall IPMNs in the last 150 IPMNs that we reported. It was 30% in the branch ducts IPMNs, so it was less than in main duct IPMNs, but it was still in the same range and I wonder why our data and your data are so much different.
I congratulate you on an excellent large series and very nicely presented.
Dr. Sauvenet: The patients of our series were French, mainly from the north of France. Only 4 to 5 surgical centers are clearly involved in the management of IPMNs in France; that is the most likely explanation for the size of the series I presented. Some patients were referred to our institution because our management is based on a multidisciplinary approach. Several gastroenterologists of our institution have a great experience of ERCP and endoscopic ultrasound in IPMN. Our pathologists are very experienced and accurate. This is another explanation for the size of the series.
Concerning the prevalence of the disease, I have no clear answer. You probably know the study recently published in Pancreas by the Mayo Clinic group; this study did not demonstrate any argument for an increased prevalence of the disease for the last 20 or 25 years.
The patients I presented had actually IPMN and not PANIN. This point was accurately discussed by our pathologists and, in all the patients included in our study, there was both a marked mucus secretion and ductal dilatation which are, to my knowledge, 2 reliable criteria to differentiate IPMN from PANIN.
When FS reveals adenoma on main duct, I do believe that an additional resection up to a total pancreatectomy should be performed in a low-risk patient with a long life expectancy, provided he accepts a possible total pancreatectomy.
Concerning the risk of malignancy in branch duct IPMN, I think that the exact risk still remains to be established. In our early experience, the risk of invasive malignancy in branch duct IPMN was 0%; it is now 9%. As you suggested, we do not know today all about this disease. We have improved our knowledge of this disease, but we have to follow very carefully the patients, those who are operated and those who are not. The risk of invasive malignancy for branch duct IPMN ranges between 5% and 20% in most of published series. Usually, it is close to 10%, but this rate could change in the future.
Dr. Bassi: I wish to congratulate you for the presentation because we absolutely need data in this field; we have no reference point for our surgical behavior, so it is very important.
I want just to stress the comment of Dr. Cameron. Twenty years ago, we probably treated some patients with “chronic pancreatitis” in reality suffering from IPMT. I feel that the data from epidemiologists about the higher incidence of pancreatic ductal cancer in chronic pancreatitis are correct, and I wonder if some cases of malignant IPMT are within this group of patients.
I think that the present paper will be outstanding when we will have the long-term follow-up of these patients. I want also to stress the problem of the denudation of the epithelium. All the recurrences we experienced were in cases in which intraoperatively the pathologists stated: “I do not see epithelium!” This is the reason for which we cannot trust on biopsies and FNA cytology. I would like also to discuss another important problem in everyday practice: patients’ information. My behavior nowadays is to put everything on the table with the patient and to present our knowledge and lack of knowledge in this field. I usually ask them to accept me free to go from simple laparotomy to total pancreatectomy.
Dr. Sauvanet: I completely agree with you.
Footnotes
Reprints will not be available from the authors.
Correspondence: Alain Sauvanet, MD, Department of Hepatopancreatobiliary Surgery, Hospital Beaujon, University Paris VII, AP-HP, 100 Bd du Général Leclerc, 92118 Clichy-Cedex, France. E-mail: alain.sauvanet@bjn.aphp.fr.
REFERENCES
- 1.Longnecker DS, Adler G, Hruban RH, et al. Intraductal papillary-mucinous neoplasms of the pancreas. In: WHO Classification of Tumors of the Digestive System. Lyon, France: IARC Press, 2000:237:–240. [Google Scholar]
- 2.Hruban RH, Takaori K, Klimstra DS, et al. An illustrated consensus on the classification of pancreatic intraepithelial neoplasia and intraductal papillary mucinous neoplasms. Am J Surg Pathol. 2004;28:977–987. [DOI] [PubMed] [Google Scholar]
- 3.Adsay NV, Merati K, Basturk O, et al. Pathologically and biologically distinct types of epithelium in intraductal papillary mucinous neoplasms: delineation of an ‘intestinal’ pathway of carcinogenesis in the pancreas. Am J Surg Pathol. 2004;28:839–848. [DOI] [PubMed] [Google Scholar]
- 4.Terris B, Ponsot P, Paye F, et al. Intraductal papillary mucinous tumors of the pancreas confined to secondary ducts show less aggressive pathologic features as compared with those involving the main pancreatic duct. Am J Surg Pathol. 2000;24:1372–1377. [DOI] [PubMed] [Google Scholar]
- 5.Kobari M, Egawa S, Shibuya K, et al. Intraductal papillary mucinous tumors of the pancreas comprise 2 clinical subtypes: differences in clinical characteristics and surgical management. Arch Surg. 1999;134:1131–1136. [DOI] [PubMed] [Google Scholar]
- 6.Sugiyama M, Izumisato Y, Abe N, et al. Predictive factors for malignancy in intraductal papillary-mucinous tumours of the pancreas. Br J Surg. 2003;90:1244–1249. [DOI] [PubMed] [Google Scholar]
- 7.Paye F, Sauvanet A, Terris B, et al. Intraductal papillary mucinous tumors of the pancreas: pancreatic resections guided by preoperative morphological assessment and intraoperative frozen section examination. Surgery. 2000;127:536–544. [DOI] [PubMed] [Google Scholar]
- 8.Gigot JF, Deprez P, Sempoux C, et al. Surgical management of intraductal papillary mucinous tumors of the pancreas: the role of routine frozen section of the surgical margin, intraoperative endoscopic staged biopsies of the Wirsung duct, and pancreaticogastric anastomosis. Arch Surg. 2001;136:1256–1262. [DOI] [PubMed] [Google Scholar]
- 9.Maire F, Couvelard A, Hammel P, et al. Intraductal papillary mucinous tumors of the pancreas: the preoperative value of cytologic and histopathologic diagnosis. Gastrointest Endosc. 2003;58:701–706. [DOI] [PubMed] [Google Scholar]
- 10.Rivera JA, Fernandez del Castillo C, Pins M, et al. Pancreatic mucinous ductal ectasia and intraductal papillary neoplasms: a single malignant clinicopathologic entity. Ann Surg. 1997;225:637–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Loftus EV, Olivares Pakzad BA, Batts KP, et al. Intraductal papillary-mucinous tumors of the pancreas: clinicopathologic features, outcome, and nomenclature. Gastroenterology. 1996;110:1909–1918. [DOI] [PubMed] [Google Scholar]
- 12.Traverso LW, Peralta EA, Ryan JA, et al. Intraductal neoplasms of the pancreas. Am J Surg. 1998;175:426–432. [DOI] [PubMed] [Google Scholar]
- 13.Salvia R, Fernandez-del Castillo C, Bassi C, et al. Main-duct intraductal papillary mucinous neoplasms of the pancreas: clinical predictors of malignancy and long-term survival following resection. Ann Surg. 2004;239:678–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sohn TA, Yeo CJ, Cameron JL, et al. Intraductal papillary mucinous neoplasms of the pancreas: an updated experience. Ann Surg. 2004;239:788–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.D'Angelica M, Brennan MF, Suriawinata AA, et al. Intraductal papillary mucinous neoplasms of the pancreas: an analysis of clinicopathologic features and outcome. Ann Surg. 2004;239:400–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bernard P, Scoazec JY, Joubert M, et al. Intraductal papillary-mucinous tumors of the pancreas: predictive criteria of malignancy according to pathological examination of 53 cases. Arch Surg. 2002;137:1274–1278. [DOI] [PubMed] [Google Scholar]
- 17.Tanaka M. Intraductal papillary mucinous neoplasm of the pancreas: diagnosis and treatment. Pancreas. 2004;28:282–288. [DOI] [PubMed] [Google Scholar]
- 18.Obara T, Maguchi H, Saitoh Y, et al. Mucin-producing tumor of the pancreas: natural history and serial pancreatogram changes. Am J Gastroenterol. 1993;88:564–569. [PubMed] [Google Scholar]
- 19.Sai JK, Suyama M, Kubokawa Y, et al. Management of branch duct-type intraductal papillary and mucinous tumor of the pancreas based on magnetic resonance imaging. Abdom Imaging. 2003;28:694–699. [DOI] [PubMed] [Google Scholar]
- 20.Sugiyama M, Abe N, Tokuhara M, et al. Magnetic resonance cholangiopancreatography for postoperative follow-up of intraductal papillary mucinous tumors of the pancreas. Am J Surg. 2003;185:251–255. [DOI] [PubMed] [Google Scholar]
- 21.Chari ST, Yadav D, Smyrk T, et al. Study of recurrence after surgical resection of intraductal papillary mucinous neoplasm of the pancreas. Gastroenterology. 2002;123:1500–1507. [DOI] [PubMed] [Google Scholar]
- 22.Fernández-del Castillo C. Surgical treatment of intraductal papillary mucinous tumors of the pancreas. J Gastrointest Surg. 2002;6:660–661. [DOI] [PubMed] [Google Scholar]
- 23.Falconi M, Salvia R, Bassi C, et al. Clinicopathological features and treatment of intraductal papillary mucinous tumour of the pancreas. Br J Surg. 2001;88:376–381. [DOI] [PubMed] [Google Scholar]
- 24.Sauvanet A, Partensky C, Sastre B, et al. Medial pancreatectomy: a multi-institutional retrospective study of 53 patients by the French Pancreas Club. Surgery. 2002;132:836–843. [DOI] [PubMed] [Google Scholar]
- 25.Sugiyama M, Atomi Y. Intraductal papillary mucinous tumors of the pancreas: imaging studies and treatment strategies. Ann Surg. 1998;228:685–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cuillerier E, Cellier C, Palazzo L, et al. Outcome after surgical resection of intraductal papillary and mucinous tumors of the pancreas. Am J Gastroenterol. 2000;95:441–445. [DOI] [PubMed] [Google Scholar]
- 27.Yamao K, Ohashi K, Nakamura T, et al. The prognosis of intraductal papillary mucinous neoplasms of the pancreas. Hepatogastroenterology. 2000;47:1129–1134. [PubMed] [Google Scholar]
- 28.Kitagawa Y, Unger TA, Taylor S, et al. Mucus is a predictor of better prognosis and survival in patients with intraductal papillary mucinous tumor of the pancreas. J Gastrointest Surg. 2003;7:12–19. [DOI] [PubMed] [Google Scholar]
- 29.Kaneko T, Nakao A, Nomoto S, et al. Intraoperative pancreatoscopy with the ultrathin pancreatoscope for mucin-producing tumors of the pancreas. Arch Surg. 1998;133:263–267. [DOI] [PubMed] [Google Scholar]