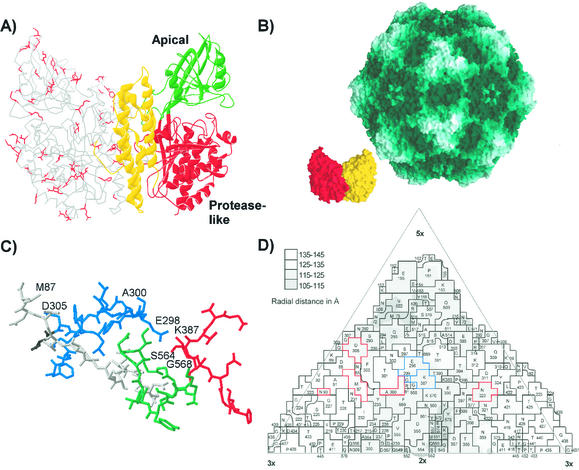
FIG. 7.
(A) Differences between the feline and canine TfR sequences mapped within the structure of the human TfR. One monomer of the ectodomain of the human TfR is shown as a ribbon diagram, while the other monomer is shown as an α-carbon tracing. The side chains of residues in the human TfR model that are unique differences between the feline and canine TfR sequences are indicated in red on one monomer. (B) Models showing the surface of the capsid of CPV (46) and of a dimer of the human TfR ectodomain (17) at the same scale. The monomers of the TfR dimer are shown in red and yellow. (C) The structure of the CPV type 2 capsid in the region that controls host range and canine TfR binding. The polypeptide chains contributed by the four VP2 monomers that make up this region of the capsid are colored differently, and the residues that are discussed in the text as controlling host range or receptor binding are labeled. (D) A road map determined by the method of Rossmann and Palmenberg (32) showing the surface exposure of VP2 residues in one asymmetric unit of the CPV type 2 capsid. The region shown is comprised of several symmetry-related VP2 subunits, and the residues are given, along with the positions in the VP2 sequence. Residues that affect receptor binding or host range and which differ naturally between FPV and CPV strains are outlined in red, whereas residues that were experimentally identified as affecting the feline or canine host ranges of the viruses are indicated in blue.