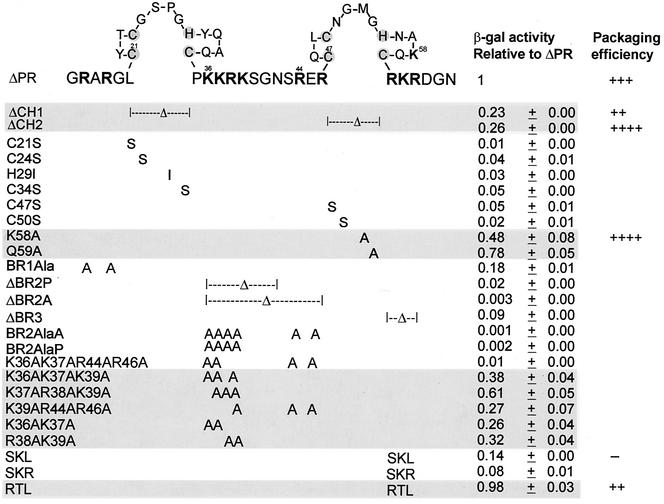
FIG. 1.
NC site-directed mutations and the resulting β-Gal activities relative to ΔPR. The amino acid sequence of NC is shown using one-letter abbreviations. Amino acid numbering is from the amino terminus of NC. The positively charged residues are indicated in bold type, and the Zn-coordinating residues within the CH boxes are shaded. β-Gal activity relative to that of ΔPR is given as the average ± standard deviation of the values for three to four independent transformants in three assays. The β-Gal activity of cells cotransformed with the RNA hybrid plasmid carrying MΨ sequences and the ΔPR protein hybrid plasmid is about 1,500 U. Mutants with β-Gal activity that was more than 20% of ΔPR are shaded. Packaging efficiencies are summarized from Table 1, Fig. 3 for the SKL mutant, or previous experiments for the RTL mutants (Table 2 in reference 31). Packaging efficiency symbols: ++, the relative packaging efficiency to ΔPR is about 0.8; +++, the relative packaging efficiency to ΔPR is about 1.0; ++++, the relative packaging efficiency to ΔPR is four- to sevenfold more than ΔPR; −, the relative packaging efficiency to ΔPR is about 1% that of ΔPR.