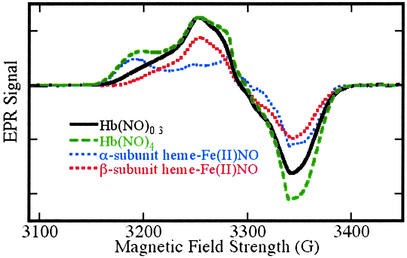
Figure 3.
EPR spectra of Fe(II)NO obtained by reductive nitrosylation of met-Hb with NO. EPR spectra were obtained at 9.29 GHz, with 10 mW incident power and field modulation of 10 G at 100 kHz. The external field was scanned over a range of 400 G in 2 min with a detection time constant of 0.128 sec. The experimental spectra shown were obtained with samples reacted with [NO]o/[heme] ratios of 0.2 (solid line) and 2 (dashed line). Reconstructions made as weighted combinations of α and β subunit spectra (dotted lines) are indistinguishable from the experimental spectra. The spectrum obtained with a [NO]o/[heme] ratio of 0.2 reflects a β vs. α subunit preference of 88 ± 8% (variance between methods was adapted from refs. 18–22). The spectrum obtained with a [NO]o/[heme] ratio of 2 reflects equal α and β subunit populations in the (Fe(II)NO)4 protein. EPR signals from residual met-Hb lie outside the field range included in the spectra presented here.