Abstract
By combining information from 2 databases, we investigated the possibility of an association between the genotype of Staphylococcus aureus causing bovine intramammary infection and dry-period cure of subclinical infection. The 1st database contained bacteriologic and cow data from a field study evaluating the efficacy in such infections of a new intramammary drycow therapy (DCT) containing tilmicosin phosphate, in comparison with a commercially available DCT containing benzathine cloxacillin. Isolates of S. aureus from that study were frozen and later independently analyzed by pulsed-field gel electrophoresis (PFGE) and macrorestriction DNA fingerprinting. The molecular information, summarized and published elsewhere, constituted the 2nd database. Data from 121 subclinically infected quarters of 92 cows from 40 herds were studied by univariate and multivariable regression analysis. Infection by an isolate of PFGE lineage group D was more likely than infection by an isolate of group A or F to be cured (P < 0.05). Cows infected by lineage group D had a higher linear somatic cell count score (LS) from the last Dairy Herd Improvement test before the dry period than did cows infected by the other lineage groups (P = 0.04). Although the probability of cure was significantly lower for cows with an LS at or above the mean of 5.7 for the study population (P = 0.05), when such a cow was infected with lineage group D, cure was significantly more likely (P < 0.001) than when it was infected by another lineage group. Significantly more (P = 0.02) of the infections treated with tilmicosin (74%) than of those treated with benzathine cloxacillin (53%) were cured, and significantly more (P = 0.05) of the infections by group D (81%) than of those by group A (57%) or group F (54%) were cured. However, there was no difference in cure rate for any PFGE genotype when tilmicosin phosphate was administered; when benzathine cloxacillin was administered, 87% of lineage group D isolates were eliminated, as compared with 46% of group A and 33% of group F isolates (P < 0.05). This research demonstrates that certain genotypes of S. aureus may naturally elicit a greater inflammatory response, yet be more susceptible to elimination by antibiotics in the dry period, than other genotypes.
Résumé
En combinant les informations contenues dans 2 bases de données, nous avons étudié la possibilité d’une association entre le génotype des isolats de Staphylococcus aureus causant des infections intramammaires et la guérison durant la période de tarissement d’infections sub-cliniques. La première base de données contenait des résultats d’analyse bactériologique et des données zootechniques sur des vaches et provenait d’une étude clinique évaluant l’efficacité d’un traitement intramammaire au tarissement (DCT) à l’aide de phosphate de tilmicosine, comparativement à un DCT commercialement disponible et contenant de la cloxacilline benzathine. Des isolats de S. aureus provenant de cette étude ont été congelés et analysés ultérieurement de manière indépendante par électrophorèse en champs pulsés (PFGE) et empreintes génomiques de l’ADN à l’aide d’enzymes de macrorestriction. Les informations moléculaires, résumées et publiées ailleurs, constituaient la 2e base de données. Les résultats de 121 quartiers infectés de manière sub-clinique provenant de 92 vaches dans 40 troupeaux ont été étudiés par analyse de régression univariée et multivariée. L’infection par un isolat avec un profil de PFGE appartenant au groupe D avait plus de chance de guérison (P < 0,05) qu’une infection avec un isolat du groupe A ou F. Les vaches infectées avec un isolat du groupe D avaient un score du compte de cellules somatiques (LS) au dernier contrôle laitier avant le tarissement plus élevé que les vaches infectées par un isolat appartenant aux autres groupes (P = 0,04). Bien que la probabilité de guérison était significativement plus faible pour les vaches avec un LS égal à la moyenne ou supérieur à la moyenne de 5,7 pour la population étudiée (P = 0,05), lorsqu’une telle vache était infectée avec un isolat du groupe D, la guérison était significativement plus probable (P < 0,001) que si elle était infectée avec un isolat d’un autre groupe. Un nombre significativement plus élevé (P = 0,02) d’infections fut guéri par utilisation de tilmicosine (74 %) plutôt qu’avec de la cloxacilline benzathine (53 %) et significativement plus d’infections (P = 0,05) par le groupe D (81 %) que celles par le groupe A (57 %) ou le groupe F (54 %) furent guéries. Toutefois, lorsque du phosphate de tilmicosine fut administré, il n’y avait pas de différence dans le taux de guérison entre les génotypes; lorsque de la cloxacilline benzathine fut administrée, 87 % des isolats du groupe D furent éliminés, comparativement à 46 % pour les isolats du groupe A et 33 % pour les isolats du groupe F (P < 0,05). Cette étude démonte que certains génotypes de S. aureus peuvent causer de manière naturelle une réponse inflammatoire plus marquée, mais peuvent être plus susceptibles que d’autres génotypes à être éliminé par les antibiotiques utilisés lors du tarissement.
(Traduit par Docteur Serge Messier)
Introduction
Elimination of existing cases of bovine intramammary infection, and prevention of new cases, routinely involves antibiotic treatment. This is particularly true for management of udder health in the dry period. Dry-cow antibiotic therapy (DCT), intramammary administration of long-acting antibiotics to all quarters of all cows at the end of lactation, is recommended by the National Mastitis Council (1). This procedure has been shown to prevent 50% to 80% of new cases, compared with not using DCT, and to eliminate existing cases caused by environmental pathogens (2). However, despite widespread adoption of DCT, quarters still do become infected throughout the dry period, and numerous cases existing at the end of lactation are not eliminated during the dry period. Elimination of subclinical infection with Staphylococcus aureus during the dry period remains particularly difficult. Cure rates vary from 20% to 80% of quarters (3–5). The self-cure rate has been as high as 25% to 38% (3).
Epidemiologic studies at the cow and quarter level have identified risk factors strongly associated with the cure of subclinical S. aureus infection (3–5). The age of the cow, the number of quarters that are infected before dry-off, the number of times a quarter has been culture-positive, and the somatic cell count (SCC) in the milk can all be used to predict the probability of bacteriologic cure. However, there is still considerable variation in cure rate, and many unknown factors may affect the probability of cure when DCT is used. As such, much attention has been paid to describing specific characteristics of S. aureus. Biotyping, phage typing, random amplified polymorphic DNA typing (RAPD), pulsed-field gel electrophoresis (PFGE), and binary typing (BT) have all been used to describe, understand, and fingerprint S. aureus (6–8). This knowledge has enhanced the ability to overcome obstacles in producing vaccines, helped to evaluate control and management practices, and assisted in the identification of novel strains of this pathogen (9–14). A reliable and reproducible technique, PFGE has a high discriminatory power. However, its drawbacks include intensive labor and expense (7,15). The results of binary typing, also a reproducible and discriminatory test, are easier to interpret. A recent study comparing PFGE and binary typing concluded that both techniques may be applied successfully for genetic analysis of S. aureus from bovine secretions (7).
The objective of this analysis was to test the null hypothesis that there is no difference between molecular types of S. aureus in the proportion of natural subclinical infections that can be cured during the dry period.
For this study, 2 databases were combined. The 1st database contained the bacteriologic and cow data from a 1999–2000 field study evaluating the efficacy of an experimental DCT containing tilmicosin phosphate (Provel, a division of Eli Lilly, Guelph, Ontario) in eliminating subclinical S. aureus intramammary infection. This new DCT was compared with a commercially available DCT containing benzathine cloxacillin (Dry-Clox; Ayerst Laboratories, Guelph), which served as a positive control. Bacteriologic results for quarter milk collected before the dry period and in the 1st month after calving, along with cow Dairy Herd Improvement (DHI) data, were readily available. Isolates from all cultures of S. aureus from the field study were frozen and later independently analyzed for molecular type by PFGE. The PFGE profiles constituted the 2nd database.
The molecular types and distribution of the S. aureus isolates had been published (8) without any knowledge of the cows and herds from which the isolates were obtained or of subsequent cure rates. Of the 288 isolates recovered from 58 farms over a 2-y period, 29 distinct PFGE types were identified and assigned to 1 of 6 groups (A through F) based on estimates of genetic relationships. Macrorestriction fingerprint patterns were analyzed by means of GelCompare II software (version 2.4; Applied Maths, Kortrijk, Belgium), and dendrograms were created with use of the Dice coefficient, the unweighted pair group method with arithmetic means, and a position tolerance of 1%. Isolates with identical restriction patterns were assigned to the same type (8). Lineage group A represented the largest genotypic group, accounting for 62% of the isolates. Group F was next largest, comprising 26% of isolates. In 59% of the herds, only a single PFGE type of S. aureus was recovered (8).
For the current analysis, the documented molecular descriptions for 121 of the S. aureus-infected quarters (8) were merged with the existing knowledge of whether the infection had been defined as cured in a particular quarter. As well, the cow and herd data associated with that quarter infection were used to further describe risk factors for cure in the dry period. The decision on which quarters to use in the analysis was based solely on the availability of complete data. Of the 288 isolates, many represented the same quarter, and some were from cows and quarters excluded from the DCT trial because of missed samples, other antibiotic treatment, or death or sale of the cow before trial completion. Others were from cows that were screened for the trial but never enrolled; thus, complete bacteriologic and risk factor data had not been collected.
Materials and methods
Herd selection and cow enrolment
The participation of herds from 3 geographic regions of Canada (Prince Edward Island, Quebec, and Ontario) and specific enrolment criteria for the cows have been reported previously (3,16). Herd selection was based on proximity to the research institution in each region to allow for regular visits by a research technician for sample collection. All mature cows that had ended at least their 1st lactation and had not received parenteral or intramammary antibiotic therapy within the last 30 d were available for enrolment. Quarter-milk samples were aseptically obtained from the enrolled cows at 4 and 2 wk before the anticipated dry-off date. If at least 1 sample was culture-positive for S. aureus, the cow was considered to be S. aureus-positive and was randomly assigned to receive either the experimental product containing tilmicosin phosphate or the commercially available product containing benzathine cloxacillin in all 4 quarters at dry-off. Quarter-milk samples were collected from all the enrolled cows on the day of dry-off.
After the dry period, quarter-milk samples were aseptically collected in the 1st, 3rd, and 4th weeks after calving from all the S. aureus-positive cows. Any isolates of S. aureus that were cultured before or after the dry period were frozen and saved. For each cow identified as having S. aureus infection and treated, DHI information from the 3 mo preceding dry-off was collected and specific information such as dry-period length and parity was recorded. The cows were housed normally throughout the study with their herd mates, and management was not altered.
Bacteriologic procedures and definition of cure
All quarter-milk samples from all study herds and regions were frozen and sent to a single laboratory (Mastitis Research Laboratory, University of Guelph). Sample collection and laboratory procedures were conducted in accordance with the recommendations of the National Mastitis Council (1) and have previously been described (3). A standard loop containing 0.01 mL of milk was plated on blood agar, where a single colony of growth of S. aureus was considered to indicate infection. A sample was defined as contaminated if 3 or more colonies of different bacteria were present after 48 h of incubation. If S. aureus was isolated from any sample before dry-off, the quarter was considered to be infected. An infection was considered to have been cured during the dry period if all 3 milk samples from the same quarter were bacteriologically negative for S. aureus in the 1st month of lactation. Isolates of S. aureus cultured from any quarter samples were reincubated in broth solution and then transferred to tryptic soy agar slants in glass tubes to be frozen and stored at −20°C.
Statistical analysis
The genotype assigned to each isolate of S. aureus was entered into the 1st database as a predictor variable for cure. This database also contained the quarter culture results along with the cow-level data. The data were checked visually by individual record and with examination of extreme values to ensure integrity. Descriptive statistics were generated with the frequency and univariate procedures and by sorting the data on various strata (region, treatment, and cure) with the use of SAS software (17). Simple differences in proportions of strains causing infections that were cured or not cured were tested by means of Fisher’s exact test.
Logistic regression for the probability of cure during the dry period for an individual quarter was modeled by fitting a generalized linear model with a logit link function and a binomial error distribution (PROC GENMOD, SAS version 8.02). The predictor variable of linear SCC score (LS) was centered by subtracting the mean of 5.7. Since mammary quarters of a cow are not independent, we used generalized estimation equations (18) to account for this correlation. The variance components at both the herd and the cow level were evaluated (PROC VARCOMP) to decide whether herd and cow effects would be considered as either random or fixed in the final model. Because the data demonstrated very little clustering in herds once the clustering within cows had been adjusted for, we excluded the fixed effect of herd in the final model. We used a compound symmetry correlation structure (equal correlation between quarters within a cow). A univariate model with each independent variable of interest was evaluated first, with all variables at P < 0.20 allowed to enter the multivariable model. Variables with P < 0.10 were allowed to stay in the final model, and the variable lineage group was forced in, because it served as the main explanatory variable in this study. Backward selection of main effects was done, and biologically meaningful 2-way interactions between significant variables were examined. Owing to the sparseness of the data relative to the model size, adding more than a single interaction proved impossible.
Results
Complete bacteriologic information to define cure of S. aureus infection, as well as molecular typing information, was available for 121 individual quarters. Descriptions of herd general management practices and practices specific to udder health control have previously been reported (3). There were 5, 65, and 51 quarters from 5, 45, and 42 cows in Prince Edward Island, Quebec, and Ontario, respectively. Descriptive statistics for the enrolled cows are shown in Table I. The average number of S. aureus-infected quarters per cow was 1.3.
Table I.
Descriptive statistics for sources of Staphylococcus aureus isolated in a dry-cow antibiotic efficacy field trial in eastern Canada in 1999–2000
| Number or score
|
|||||
|---|---|---|---|---|---|
| Variable | Total or mean | Range or s | Prince Edward Island | Quebec | Ontario |
| Herds | 40 | 3 | 16 | 21 | |
| Cows per herd | 64 | 27–168 | 142 | 48 | 78 |
| Cows enrolled | 92 | 5 | 45 | 42 | |
| Lactations | 2.9 | 1–8 | 2.8 | 3.0 | 2.8 |
| Dry-period length (d) | 66 | 18.5 | 71.8 | 65.3 | 66.3 |
| Last LS before dry-off (and 95% CI per region) | 5.7 | 1.3 | 6.2 | 5.9 | 5.4 |
| Infected quarters | 121 | (5.1 to 7.3) | (5.6 to 6.2) | (5.0 to 5.7) | |
| Infected per cow | 1.3 | 5 | 65 | 51 | |
| DCT with tilmicosin | 53 | 1.0 | 1.4 | 1.2 | |
| DCT with cloxacillin | 68 | ||||
s — standard deviation; LS — linear somatic cell count score, from Dairy Herd Improvement test; CI — confidence interval; DCT — intramammary dry-cow antibiotic therapy administered at dry-off
The 121 isolates of S. aureus had 18 distinct macrorestriction profiles, previously assigned to 3 lineage groups (A, D, and F) (8): 60% of the isolates belonged to group A, 22% to group D, and 18% to group F. Types A and F were isolated from all 3 geographic regions, whereas type D was not isolated from Prince Edward Island (Table II). In most herds, only 1 lineage group was isolated from all cows enrolled, and no herd demonstrated all 3 groups; however, in 5 herds (12%), 2 groups were isolated (Table III). Cows infected by lineage group D had a higher LS on the last DHI test than did cows infected by lineage group A or F (P = 0.04).
Table II.
Descriptive data for pulsed-field gel electrophoresis (PFGE) genotypes of the S. aureus isolates from 92 cows subclinically infected at the end of lactation (8)
| Number of isolates (and number of cows)
|
|||||
|---|---|---|---|---|---|
| Lineage group of S. aureus | Total number (and %) of isolates | Number of distinct genotypes recognized | Prince Edward Island (5) | Quebec (45) | Ontario (42) |
| A | 72 (60) | 9 | 1 | 40 | 31 |
| D | 27 (22) | 3 | 0 | 21 | 6 |
| F | 22 (18) | 6 | 4 | 4 | 14 |
| Total | 121 | 18 | 5 | 65 | 51 |
Table III.
Distribution of PFGE lineage groups among the 40 herds
| Number (and %) of herds
|
|||
|---|---|---|---|
| Province | Total number of herds | With 1 lineage group identified | With 2 lineage groups identified |
| Prince Edward Island | 3 | 3 (100) | 0 (0) |
| Quebec | 16 | 13 (81) | 3 (19) |
| Ontario | 21 | 19 (90) | 2 (10) |
| Total | 40 | 35 (88) | 5 (12) |
Table IV demonstrates the univariate effect of treatment and lineage group on cure of S. aureus infection. Significantly more (P = 0.02) of the infections treated with tilmicosin (74%) than of those treated with benzathine cloxacillin (53%) were cured. In addition, significantly more (P = 0.05) of the infections with S. aureus of lineage group D (81%) than of those with group A (57%) or group F (54%) were cured. This effect of lineage group was influenced greatly by treatment received at dry-off (Figure 1): there was no difference in cure rate between lineage groups A, D, and F among the quarters treated with tilmicosin (71%, 75%, and 80%, respectively), whereas group D infections were significantly more likely (P = 0.001) to be cured than were group A and F infections among the quarters treated with benzathine cloxacillin (87%, 46%, and 33%, respectively).
Table IV.
Univariate effect of DCT and lineage group on the rate of bacteriologic cure during the dry period
| Variable | Number (and %) of infections cureda |
|---|---|
| Overall | 75/121 (62) |
| Antibiotic | |
| Tilmicosin | 39/53 (74b) |
| Cloxacillin | 36/68 (53c) |
| Lineage group | |
| A | 41/72 (57b) |
| D | 22/27 (81c) |
| F | 12/22 (54b) |
An infection was considered to have been cured during the dry period if all 3 milk samples from the same quarter that had been culture-positive before the dry period were bacteriologically negative for S. aureus in the 1st month of the next lactation. Proportions with different superscripts differed significantly (P < 0.05) by treatment or lineage group.
Figure 1.
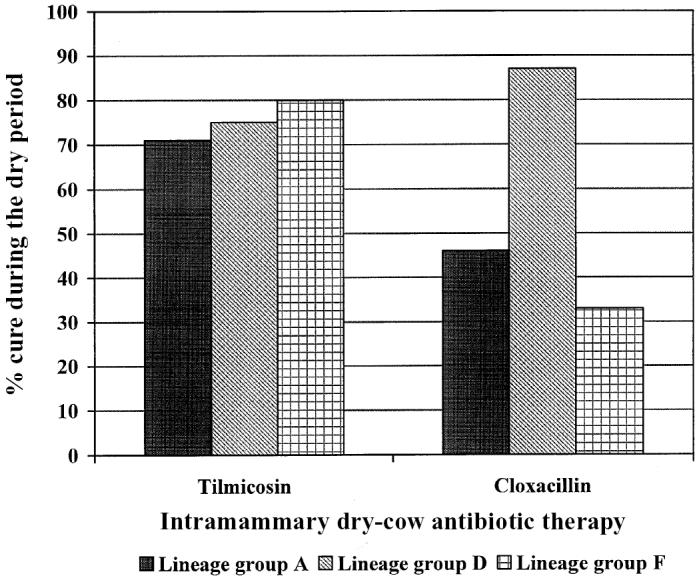
Influence of antibiotic on the rate of dry-period cure with intramammary treatment of infection by 121 isolates of Staphylococcus aureus belonging to 3 lineage groups as determined by pulsed-field gel electrophoresis and macrorestriction DNA fingerprint analysis.
Interpretation of the final logistic regression model (Table V) shows the influence of specific variables on the probability of cure in an infected quarter. While controlling for other variables in the model, infections in quarters of 1st- and 2nd-lactation cows had a significantly higher probability (P = 0.05) of cure than did those of 3rd-lactation and older animals. The probability of cure was significantly lower (P = 0.05) for cows with an LS at or above the mean of 5.7. The main effects of lineage group on cure were not significant with dry LS centered in the final model, although there was still a strong tendency for cure of infections by group D organisms compared with infections by group A organisms. However, there was a significant interaction between lineage group and LS on the last DHI test: when the LS was at least the mean value and the quarter was infected with group D S. aureus, cure was significantly more likely (P < 0.001). The interaction between lineage group and treatment was significant in the multivariable logistic regression model. However, inclusion of both interaction terms (lineage group by dry LS and lineage group by treatment) resulted in a model that was overfitted with nonsensical parameter estimates and standard errors. Therefore, we decided to leave only lineage group by dry LS in the final model.
Table V.
Final logistic regression model determining the probability of cure of an infected quarter during the dry period
| Predictor variable | β estimate | Standard error | 95% CI | P-value |
|---|---|---|---|---|
| Parity | ||||
| 1 | 1.12 | 0.58 | −0.01 to 2.3 | 0.05 |
| 2 | 1.09 | 0.56 | −0.01 to 2.2 | 0.05 |
| 3+ | Ref | — | — | — |
| Lineage group | ||||
| A | Ref | — | — | — |
| D | 1.25 | 0.68 | −0.09 to 2.6 | 0.06 |
| F | −0.45 | 0.61 | −1.7 to 0.75 | 0.46 |
| Antibiotic | ||||
| Tilmicosin | 0.92 | 0.45 | 0.03 to 1.8 | 0.04 |
| Cloxacillin | Ref | |||
| Last LS before dry-off | ||||
| Centereda | −0.56 | 0.29 | −1.1 to 0.01 | 0.05 |
| × lineage D | 1.77 | 0.46 | 0.88 to 2.7 | < 0.001 |
| × lineage F | −0.10 | 0.45 | −0.99 to 0.77 | 0.81 |
Ref — referent comparison group for β estimate
To represent a cow with the mean LS of 5.7
Discussion
Several studies have examined factors at both the cow and the quarter level that might predict cure in the dry period of S. aureus infection (3–5). As SCC, age of the cow, or number of quarters infected increase, the probability of cure decreases. The repeatability and strength of these associations across studies have influenced culling and treatment decisions in herds. However, none of those studies examined genotypes. Inclusion of genotype, a unique and advantageous aspect of our study, was achievable only by merging the independently obtained and previously published molecular descriptions of the isolates with the quarter bacteriologic and cow data.
The influence of strain type on changes in SCC and on clinical mastitis in lactation has been studied more extensively. One of the most recent studies found no significant differences among S. aureus strains and concluded that the degree of parenchymal injury induced by S. aureus infection is affected by factors other than strain type (13). Similarly, strain information has been used to study the epidemiologic features of S. aureus infection, characterizing its spread, and to implement control practices (19). Genotyping has also been helpful in demonstrating the contagiousness of the pathogen (6,12). Previous research has found only a limited number of S. aureus genotypes in any given herd, which might be attributable to quarter-to-quarter spread of the pathogen (8,9,19). Mathematical modeling has also indicated that this is the most likely explanation for spread of subclinical S. aureus mastitis (19).
Our study supports that explanation. Overall, 88% of the 40 herds had strains from a single lineage group in the cows subclinically infected with S. aureus. However, we cannot say that only 1 genotype was isolated from the same herd. There was considerable genetic heterogeneity among the isolates (Table II). For our analysis, we used 121 isolates from 40 farms out of 288 isolates from 58 farms in PFGE research by Agriculture Canada (8). In the original dataset, 59% of the farms had a single genotype identified, 24% had 2 genotypes, and 10% had 3 genotypes. We would expect similar heterogeneity in the subset of isolates that we used.
The current experiment tested the hypothesis that no difference would exist in the rate of cure by DCT of subclinical infection by different S. aureus genotypes. In interpreting the results, one should bear in mind that such purposively selected convenience samples may not be representative of a larger, truly random population of dairy cows. None the less, the results demonstrate that the lineage group of the S. aureus isolates, as determined by PFGE, significantly influenced the probability of cure during the dry period. Although there are contradictory reports on the pathogenicity of different S. aureus strains (13), it has been suggested that the organism’s virulence may be strain-dependent. Therefore, knowing the strain causing an infection and making informed treatment or culling decisions on the basis of that information may lead to advances in control and management of S. aureus infection (7,13).
The only surrogate measure of virulence available in this experiment was the LS from the last DHI test before the dry period. The lineage group of S. aureus with the highest cure rate had a significantly higher LS, which may indicate the importance of virulence in cure. We explored this possibility in the multivariable model, as it would contradict previous research findings that, in general, an increased SCC negatively influences the probability of cure of S. aureus infection in the dry period. What this study has demonstrated is that genotypes of S. aureus within a particular lineage group may naturally cause a higher LS than other genotypes, yet increase the probability of cure. Studies in natural populations of S. aureus have identified considerable genetic heterogeneity, and data suggest that certain strain types are more likely to produce particular virulence factors (10). This should be the subject of further research.
A larger field trial of the experimental intramammary treatment previously determined its efficacy at eliminating S. aureus (3). The current analysis, using only a subset of cows and infected quarters, continued to show a significant difference between tilmicosin treatment and the positive control treatment with benzathine cloxacillin. Of more interest in this study was the interaction exposed between intramammary therapy and PFGE lineage group on rate of cure. Further investigation of this effect would best be served in a more detailed exploration of antibiotic resistance patterns of the isolates involved. The patterns of the entire set of 288 S. aureus isolates have previously been described (8): resistance to penicillin was the most common, affecting 9.9% of the isolates, whereas only 0.9% (2) were resistant to tilmicosin.
Despite the unique findings of this research, it will remain a challenge to incorporate this information into applicable recommendations at the herd level. Even with the previous knowledge of risk factors for cure of S. aureus infection (SCC, age, and number of quarters infected), incorporation of this information into day-to-day herd management decisions has not been widely adopted. As such, expecting that further diagnostic tests and expense would be readily adopted by the industry is not realistic. However, this study offers more insight and understanding into the area of cure of subclinical S. aureus infection and may allow more meaningful decisions by producers willing and prepared to make the most informed decisions. The availability, cost, and turn-around time of the test will further influence its adoption by producers. None the less, from a research perspective, this study has further elucidated the pathophysiology and epidemiology of cure of subclinical S. aureus infection in dairy herds, which will help dairy advisers and veterinarians bring the most up-to-date information to producers.
In conclusion, on the basis of our findings, we rejected the hypothesis that there is no difference in the probability of cure during the dry period of natural subclinical infection by various PFGE genotypes of S. aureus. Overall, the cure rate was higher for isolates of lineage group D than for isolates of groups A and F. Therefore, determining the predominant strain in a herd may augment the management and control of S. aureus infection, especially with respect to cure during the dry period, since we found an interaction between lineage group and the last LS before dry-off. Historically, an increased SCC in the milk of cows infected with S. aureus has strongly predicted a decreased probability of cure. This research demonstrates that certain genotypes of S. aureus may naturally elicit a greater inflammatory response, yet be more susceptible to elimination by antibiotics in the dry period, than other genotypes.
Acknowledgements
The authors gratefully acknowledge the clinicians and research technicians associated with each veterinary college who identified and enrolled herds and who collected the milk samples and herd data. We also thank Henrik Stryhn for valuable statistical advice.
References
- National Mastitis Council. Laboratory Handbook on Bovine Mastitis, rev ed. Madison, Wisconsin: NMC, 1999.
- Eberhart RJ. Management of dry cows to reduce mastitis. J Dairy Sci. 1986;69:1721–1732. doi: 10.3168/jds.S0022-0302(86)80591-4. [DOI] [PubMed] [Google Scholar]
- Dingwell RT, Leslie KE, Duffield TF, et al. Efficacy of intramammary tilmicosin and risk factors for cure of Staphylococcus aureus infection in the dry period. J Dairy Sci. 2003;86:159–168. doi: 10.3168/jds.S0022-0302(03)73596-6. [DOI] [PubMed] [Google Scholar]
- Nickerson SC, Owens WE, Fox LK, Scheifinger CC, Shryock TR, Spike TE. Comparison of tilmicosin and cephapirin as therapeutics for Staphylococcus aureus mastitis at dry-off. J Dairy Sci. 1999;82:696–703. doi: 10.3168/jds.S0022-0302(99)75286-0. [DOI] [PubMed] [Google Scholar]
- Sol J, Sampimon OC, Snoep JJ, Schukken YH. Factors associated with bacteriological cure after dry cow treatment of subclinical staphylococcal mastitis with antibiotics. J Dairy Sci. 1994;77:75–79. doi: 10.3168/jds.S0022-0302(94)76930-7. [DOI] [PubMed] [Google Scholar]
- Zadoks RN, Schukken YH, Barkema H. Staphylococcus aureus: fingerprinting the culprit. In: Proc Annu Meet Natl Mast Counc 2000:79–93.
- Zadoks R, van Leeuwen W, Barkema H, et al. Application of pulsed-field gel electrophoresis and binary typing as tools in veterinary clinical microbiology and molecular epidemiologic analysis of bovine and human Staphylococcus aureus isolates. J Clin Microbiol. 2000;38:1931–1939. doi: 10.1128/jcm.38.5.1931-1939.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabour PM, Gill JJ, Lepp D, et al. Molecular typing and distribution of Staphylococcus aureus isolates in eastern Canadian dairy herds. J Clin Microbiol. 2004;42:3449–3455. doi: 10.1128/JCM.42.8.3449-3455.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annemüller C, Lämmler C, Zschöck M. Genotyping of Staphylococcus aureus isolated from bovine mastitis. Vet Microbiol. 1999;69:217–224. doi: 10.1016/s0378-1135(99)00117-0. [DOI] [PubMed] [Google Scholar]
- Fitzgerald JR, Hartigan PJ, Meaney WJ, Smyth CJ. Molecular population and virulence factor analysis of Staphylococcus aureus from bovine intramammary infections. J Appl Microbiol. 2000;88:1028–1037. doi: 10.1046/j.1365-2672.2000.01071.x. [DOI] [PubMed] [Google Scholar]
- Guidry A, Fattom A, Patel A, O’Brien C, Shepherd S, Lohuis J. Serotyping scheme for Staphylococcus aureus isolated from cows with mastitis. Am J Vet Res. 1998;59:1537–1539. [PubMed] [Google Scholar]
- Joo YS, Fox LK, Davis WC, Bohach GA, Park YH. Staphylococcus aureus associated with mammary glands of cows: genotyping to distinguish different strains among herds. Vet Microbiol. 2001;80:131–138. doi: 10.1016/s0378-1135(00)00381-3. [DOI] [PubMed] [Google Scholar]
- Middleton JR, Fox LK, Gay JM, Tyler JW, Besser TE. Influence of Staphylococcus aureus strain-type on mammary quarter-milk somatic cell count and N-acetyl-beta-D-glucosaminidase activity in cattle from eight dairies. J Dairy Sci. 2002;85:1133–1140. doi: 10.3168/jds.S0022-0302(02)74175-1. [DOI] [PubMed] [Google Scholar]
- Smith TH, Fox LK, Middleton JR. Outbreak of mastitis caused by one strain of Staphylococcus aureus in a closed dairy herd. JAVMA. 1998;212:553–556. [PubMed] [Google Scholar]
- Montesinos I, Salido E, Delgado T, Cuervo M, Sierra A. Epidemiologic genotyping of methicillin-resistant Staphylococcus aureus by pulsed-field gel electrophoresis at a university hospital and comparison with antibiotyping and protein A and coagulase gene polymorphisms. J Clin Microbiol. 2002;40:2119–2125. doi: 10.1128/JCM.40.6.2119-2125.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingwell RT, Duffield TF, Leslie KE, et al. The efficacy of intramammary tilmicosin at drying-off, and other risk factors for the prevention of new intramammary infections during the dry period. J Dairy Sci. 2002;85:3250–3259. doi: 10.3168/jds.S0022-0302(02)74413-5. [DOI] [PubMed] [Google Scholar]
- SAS User’s Guide: Statistics, release 8.01, Cary, North Carolina: SAS Institute, 2000.
- Liang KY, Zeger S. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73:13–22. [Google Scholar]
- Lam TJGM, Lipman LJA, Schukken YH, Gaastra W, Brand A. Epidemiological characteristics of bovine clinical mastitis caused by Staphylococcus aureus and Escherichia coli studied by DNA fingerprinting. Am J Vet Res. 1996;57:39–42. [PubMed] [Google Scholar]


