Abstract
Liu et al (Science, 304:1021-4) report that NMDA receptors containing NR2A and NR2B subunits are selectively coupled to long-term potentiation (LTP) and long-term depression (LTD) respectively. Because NR2B (but not NR2A) receptors occur outside synapses, and can be activated by glutamate spillover, this principle may underlie synaptic homeostasis.
Main Text
Liu et al (1) recently showed that blockade of NMDA receptors containing either NR2A or NR2B subunits leads to a selective defect in either long-term potentiation (LTP) or long-term depression (LTD) respectively. This report provides an elegant demonstration of complementarity of function of the receptor subtypes (2). We would like to draw attention to a potentially important implication of the results for network behavior. NR2A-containing receptors, unlike NR2B-containing receptors, are located almost exclusively within synapses (3-5). Therefore, the balance of LTP and LTD in a cell could reflect the degree to which synaptic, as opposed to extrasynaptic, receptors are activated. Liu et al modestly omitted to refer to a previous study from the same group supporting precisely this principle (6).
Taken together with recent evidence that extrasynaptic spillover of glutamate is detected exclusively by NR2B-containing NMDA receptors (7-9), these findings provide a novel mechanism for homeostatic regulation of excitatory transmission (10) and for sharpening pattern storage in the neuronal network. An elevation in ambient glutamate, released from multiple synapses and sensed by extrasynaptic NR2B-containing receptors, should trigger widespread LTD if accompanied by neuronal depolarization (Fig. 1). This does not preclude induction of LTP at synapses where glutamate is released and opens synaptic NR2A-containing receptors. The higher affinity of NR2B- than NR2A-containing receptors for glutamate (11) is well suited to their proposed role in weakening transmission as a function of heterosynaptic activity.
Figure 1.
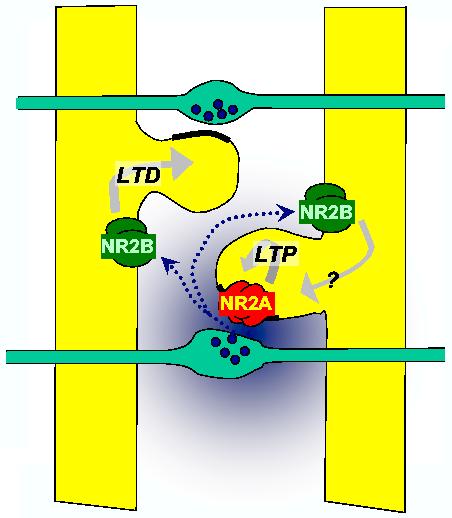
NR2B-containing receptors (unlike NR2A-containing receptors) occur in the extrasynaptic membrane. Glutamate escaping from synapses selectively activates NR2B-containing receptors (dotted arrow). The selective coupling of NR2A- and NR2B-containing receptors to LTP and LTD respectively (Liu et al) provides a mechanism for homeostatic plasticity if homosynaptic LTP is accompanied by heterosynaptic LTD.
Differential activation of NR2A- and NR2B-containing receptors by synaptic and extrasynaptic glutamate also has distinct consequences for gene transcription (12). Finally, because the relative density of synaptic and extrasynaptic NR2A- and NR2B-containing receptors changes with age (3, 13, 14), their complementary roles in synaptic plasticity may be developmentally regulated.
References
- 1.Liu L, et al. Science. 2004;304:1021. doi: 10.1126/science.1096615. [DOI] [PubMed] [Google Scholar]
- 2.Bliss TVP, Schoepfer R. Science. 2004;304:973. doi: 10.1126/science.1098805. [DOI] [PubMed] [Google Scholar]
- 3.Stocca G, Vicini S. J. Physiol. 1998;507:13. doi: 10.1111/j.1469-7793.1998.013bu.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tovar KR, Westbrook GL. J. Neurosci. 1999;19:4180. doi: 10.1523/JNEUROSCI.19-10-04180.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Steigerwald F, et al. J. Neurosci. 2000;20:4573. doi: 10.1523/JNEUROSCI.20-12-04573.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lu HC, Gonzalez E, Crair MC. Neuron. 2001;32:619. doi: 10.1016/s0896-6273(01)00501-3. [DOI] [PubMed] [Google Scholar]
- 7.Dalby NO, Mody I. J. Neurophysiol. 2003;90:786. doi: 10.1152/jn.00118.2003. [DOI] [PubMed] [Google Scholar]
- 8.Scimemi A, Fine A, Kullmann DM, Rusakov DA. J. Neurosci. 2004;24:4767. doi: 10.1523/JNEUROSCI.0364-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lozovaya NA, et al. J. Physiol. 2004 [Google Scholar]
- 10.Turrigiano GG, Nelson SB. Nat Rev Neurosci. 2004;5:97. doi: 10.1038/nrn1327. [DOI] [PubMed] [Google Scholar]
- 11.Kutsuwada T, et al. Nature. 1992;358:36. doi: 10.1038/358036a0. [DOI] [PubMed] [Google Scholar]
- 12.Hardingham GE, Fukunaga Y, Bading H. Nature Neurosci. 2002;5:405. doi: 10.1038/nn835. [DOI] [PubMed] [Google Scholar]
- 13.Kirson ED, Yaari Y. J. Physiol. 1996;497:437. doi: 10.1113/jphysiol.1996.sp021779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li JH, et al. Eur. J. Neurosci. 1998;10:1704. doi: 10.1046/j.1460-9568.1998.00169.x. [DOI] [PubMed] [Google Scholar]


