FIG. 6.
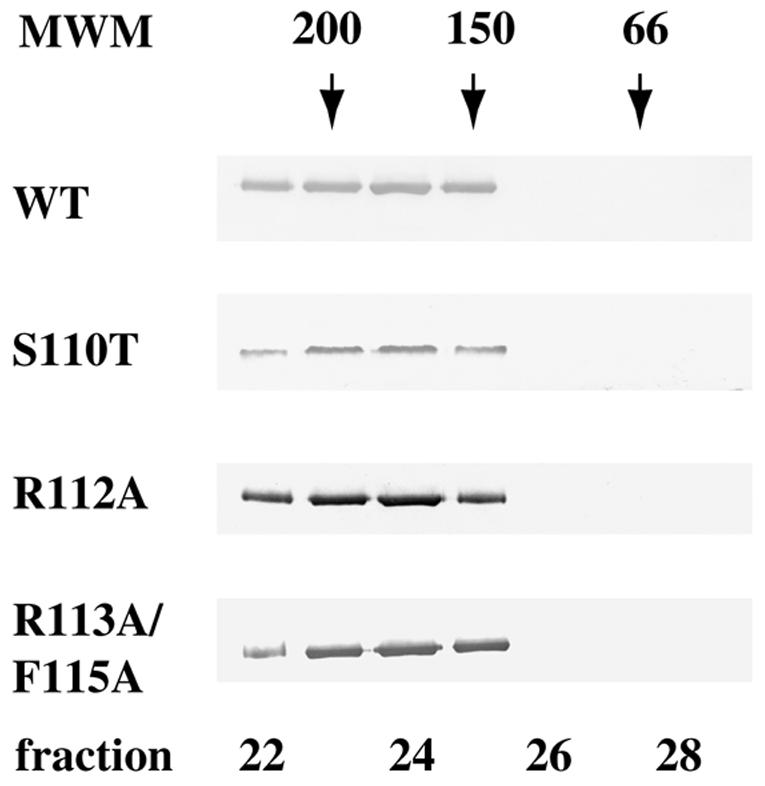
Mutations in helicase motif Ia do not alter the dimerization state of UL9 protein. A 100 nM solution of UL9 protein was analyzed by gel filtration using a Superose 12HR column (Pharmacia). Fifty 0.5-ml fractions were collected, and a 15-μl portion of each fraction was subjected to SDS-PAGE and Western blot analysis with anti-UL9 rabbit polyclonal serum R250. Wild-type (WT) and mutant UL9 proteins peak in fractions 23 to 25, corresponding to a dimeric state. The Western blots were developed for a short period of time in order to visualize only the peak fractions; however, with longer exposures, some trailing material was observed (up to fraction 27). The positions of gel filtration molecular mass markers (MWM) (Sigma) (amylase [200 kDa], alcohol dehydrogenase [150 kDa], and BSA [66 kDa]) are depicted above the Western blot strips.
