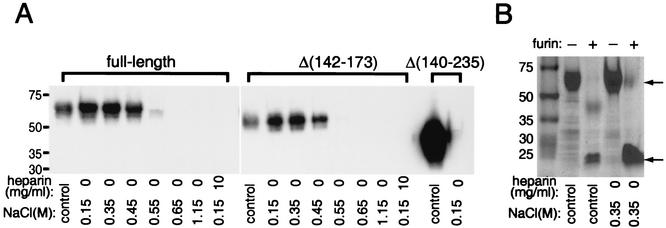
FIG. 5.
Heparin binding properties of full-length and mutated forms of MC54L. MC54L proteins that were full length or lacked amino acids 142 to 173 or 140 to 235 were expressed individually in BS-C-1 cells by recombinant vaccinia viruses and purified by metal affinity chromatography. (A) Except for the control lanes, recombinant MC54L proteins were incubated with 30 μl of heparin-agarose in the presence of various concentrations of NaCl or heparin (indicated below the lanes). Proteins that bound to heparin-agarose were eluted after extensive washing and detected by Western blotting with a MAb against the polyhistidine tag. In the control lanes, approximately half of the material that was applied to the heparin-agarose was directly run on the gel and analyzed by Western blotting. (B) Full-length MC54L protein was incubated with (+) or without (−) recombinant furin, incubated with 30 μl of heparin-agarose in the presence of 0.35 M NaCl, and analyzed by SDS-PAGE and Coomassie blue staining. Control lanes have the same meaning as in panel A. The arrows point to the full-length (top) and C-terminal fragment (bottom) forms of the MC54L protein. The values on the left indicate the mobilities and masses in kilodaltons of marker proteins.