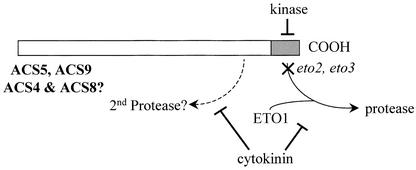
Figure 10.
Model for the Regulation of ACS Protein Stability.
The C-terminal domains of ACS5 and ACS9 (and possibly also ACS4 and ACS9, because their C-terminal domains are highly similar) acts as targets for an unknown protease. The alteration of this domain caused by either the eto2 or the eto3 mutation disrupts the targeting of the protein to the proteolytic machinery. Interaction with the ETO1 protein is required for efficient turnover of the proteins. In addition, phosphorylation of the C-terminal domain may block its function as a proteolytic tag. Cytokinin acts at least partially to block the targeting of the enzyme by this domain for proteolysis. However, the observation that the eto2 mutant protein is partially stabilized by cytokinin treatment suggests that it also works through a distinct degradation pathway. See text for more details.