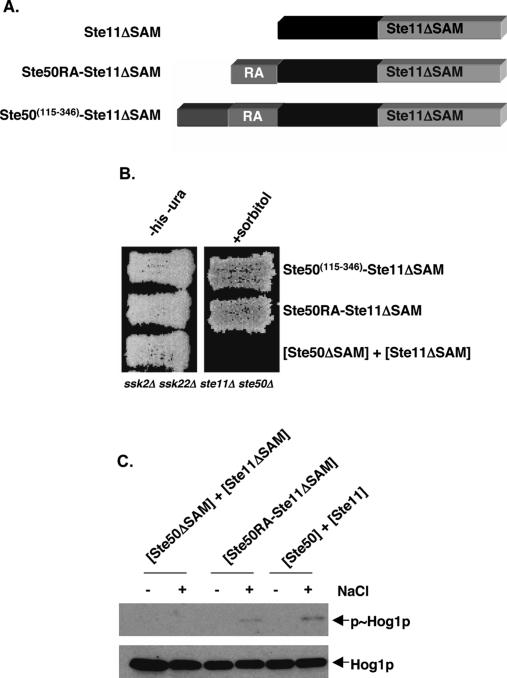
Figure 3.
Fusion of the RA domain-containing fragment of Ste50p bypasses the requirement for an interaction between the SAM domains of Ste11p and Ste50p. (A) Schematic representation of the constructs encoding fusions of the Ste50p RA domain-containing fragments and the Ste11pΔSAM fragment. (B) Yeast cells (ste11Δ ste50Δ ssk2Δ ssk22Δ) transformed with STE11 and STE50 constructs as indicated were assayed for their ability to activate the HOG pathway by testing their ability to grow on hyperosmotic medium. (−his − ura) Synthetic dextrose histidine and uracil drop-out medium; (+sorbitol) selective medium with 1.5 M sorbitol. (C) Western blot analysis of protein extracts of cells as in B—either treated (+) or untreated (−) with NaCl at 0.5 M for 5 min before the extraction of protein samples—was performed with either anti-phospho-p38 antibody to detect phosphorylated Hog1p (p∼Hog1p) or with anti-p38 antibody to detect the total amount of Hog1p.