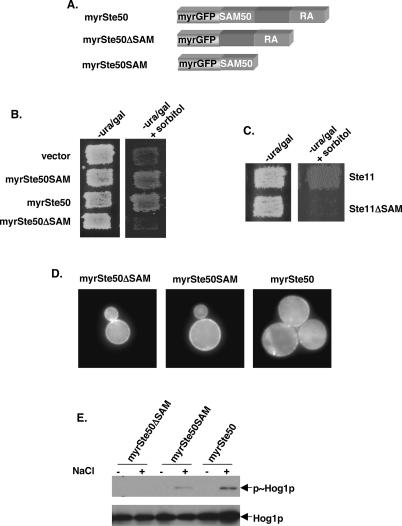
Figure 4.
Plasma-membrane targeting of the Ste50p SAM domain complements Ste50p function in the HOG pathway. (A) Schematic representation of the constructs encoding fusions of the GFP carrying a myristoylation signal and various fragments of Ste50p. (B) Yeast cells (ste50Δ ssk2Δ ssk22Δ), transformed with either the vector control or constructs encoding various fragments of Ste50p with a myristoylation signal, were assayed for the ability to grow on hyperosmotic media. (−ura/gal) Synthetic uracil drop-out with galactose as carbon source; (+sorbitol) with 1.5 M sorbitol. (C) Yeast cells (ste11Δ ste50Δ ssk2Δ ssk22Δ) cotransformed with a myristoylated Ste50p SAM domain in combination with either the wild-type Ste11p or the Ste11pΔSAM construct were assayed for the ability to grow on hyperosmotic medium. (D) Plasma membrane localization of myristoylated GFP fusion of the SAM domain and the RA-like domain of Ste50p, as well as the full-length Ste50p in ste50Δ ssk2Δ ssk22Δ cells. (E) Western blot analysis of protein extracts of cells as in B, for detecting Hog1p phosphorylation as described in Figure 3.