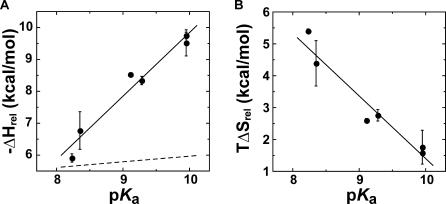
Figure 11. Dependence of Changes in Enthalpy and Entropy of Binding of a Series of meta- and para-Substituted Fluorophenolates to pKSI D40N on p K a .
(A) The relative value of ΔH binding as a function of p K a (uncorrected for the enzyme ionization enthalpy, which is constant across the series of phenolates) has a slope (solid line) of −2.0 ± 0.2 kcal/mol/p K a unit. The dotted line is the relative dependence of −ΔG on p K a from Figure 6 for comparison (−0.2 kcal/mol/p K a unit).
(B) The relative TΔS binding as a function of p K a has a slope of 2.0 ± 0.3 kcal/mol.