Abstract
Insertion of an extracorporeal left ventricular assist device for temporary ventricular support via median sternotomy can be challenging in patients with a history of cardiac surgery, because these patients often poorly tolerate the lengthy dissection and cardiac manipulation necessary for exposure of the left ventricular apex and ascending aorta. Our approach, transdiaphragmatic left ventricular inflow cannulation with return through a graft sewn to the supraceliac aorta, can be accomplished through a left upper-quadrant abdominal incision without entering the peritoneal cavity, mediastinum, or left hemithorax. Repeated sternotomy and mediastinal dissection are thus avoided.
To our knowledge, this report documents the 1st use of this technique for insertion of an extracorporeal pulsatile ventricular assist device for temporary ventricular support.
Keywords: Cardiac surgical procedures, off-pump, left ventricular assist device, thoracotomy
Placement of a temporary left ventricular assist device (LVAD) in patients who have a history of cardiac surgery via median sternotomy can be challenging. This procedure, though potentially lifesaving to these desperately ill patients, still has a mortality rate of nearly 35%. Although the initiation of circulatory assistance can successfully normalize depressed cardiac output, the magnitude of the surgical procedure is often prohibitive. Patients with hemodynamic instability necessitating support often tolerate conventional assist device implantation poorly because of the lengthy dissection and cardiac manipulation necessary for exposure of the left ventricular apex and ascending aorta. Initiating cardiopulmonary bypass (CPB) using groin cannulation can facilitate this dissection, but prolonged extracorporeal circulation may exacerbate end-organ dysfunction in these gravely ill patients. In addition, many of these patients have atherosclerosis involving the femoral arteries, which further limits the usefulness of peripheral cannulation and median sternotomy.
Transdiaphragmatic left ventricular inflow cannulation with return through a graft sewn to the supraceliac aorta can be accomplished through a left upper-quadrant abdominal incision without entering the peritoneal cavity, mediastinum, or left hemithorax. The technique avoids repeated sternotomy and mediastinal dissection and can, in many cases, be performed without the aid of CPB.
History
A 72-year-old man with a history of coronary artery disease and peripheral vascular disease went to another institution for treatment of unstable angina. He had a history of hypertension and tobacco use and had undergone coronary artery bypass surgery 12 years earlier. He was taken to the cardiac catheterization laboratory, where a diagnostic angiogram showed severe diffuse coronary artery disease and occlusion of all grafts. Percutaneous interventions were attempted in 2 lesions in the left anterior descending and circumflex coronary arteries, which resulted in the occlusion of both vessels and an acute myocardial infarction with resulting hemodynamic compromise. An intra-aortic balloon pump was therefore inserted. The patient's condition was stabilized, and he was transferred to our institution in anuric renal failure. His hemodynamic status remained marginal. Shortly after arrival, he developed abdominal tenderness. Laparotomy revealed an ischemic right colon, and a right hemicolectomy was performed. Over the next 10 days, the patient recovered from the surgical procedure but remained intubated and required hemodialysis. His hemodynamic status became further compromised despite intra-aortic balloon pumping and administration of high-dose epinephrine and milrinone (cardiac index, 1.7 L/min/m2; pulmonary artery pressure, 56/30 mmHg; pulmonary capillary wedge pressure, 26 mmHg; and mixed venous oxygen saturation, 51%). Nevertheless, he remained neurologically intact, and both he and his family expressed a desire to proceed with aggressive treatment.
Hospital Course
Surgical Technique. The patient underwent surgery in April 2005. In the operating room, a generous left upper-quadrant incision was made 2 cm below the costal margin and was extended just beyond the midline. A retroperitoneal dissection was performed, with efforts to avoid entering the abdomen. The supraceliac aorta was identified, and the diaphragmatic crura were dissected away, which readily exposed an adequate segment of aorta. The retroperitoneal dissection exposed the undersurface of the diaphragm, through which the borders of the heart could be palpated. A small diaphragmatic window was constructed to expose the inferior wall of the left ventricle.
After systemic heparin was administered, arterial and venous cannulas were inserted into the right femoral artery and vein in the event that CPB became necessary. A side-biting vascular clamp was applied to the supraceliac aorta, and the outflow graft of a Thoratec® Ventricular Assist Device (VAD) System (Thoratec Corporation; Pleasanton, Calif) was sutured in place. This challenging surgical anastomosis was performed with conventional instruments and hand-held retractors. The sutured anastomosis was reinforced with a Teflon felt strip and sealed with BioGlue® (CryoLife, Inc.; Kennesaw, Ga) to ensure hemostasis.
The inferior aspect of the heart was then examined to determine the best location for the inflow cannula. With the use of transesophageal echocardiography for guidance, a metal spinal needle was inserted through the inferior wall into the left ventricular cavity. A point lateral to the ventricular septum and medial to the papillary muscles was identified as the optimal location for the inflow cannula, and 4 pledgeted polypropylene sutures were placed radially around the intended cannulation site and through the cannula sewing ring. β-Blockers were administered systemically to slow the heart rate and decrease the vigor of ventricular contraction. A cruciate incision was made in the inferior left ventricular wall, and the cannula was inserted. The 4 sutures were tied, and additional sutures were placed radially to ensure hemostasis. The flanged sewing ring on the inflow cannula facilitated hemostasis during these maneuvers, and there was very little blood loss. Transesophageal echocardiography confirmed adequate positioning of the inflow cannula. The back ends of both cannulas were tunneled through the subcutaneous tissue, brought out through the skin, and secured. After aggressive de-airing, the cannulas were connected to the inlet and outlet ports of the LVAD, and ventricular support was successfully initiated (Fig. 1). Hemostasis was achieved in the retroperitoneum, and the abdominal wall was closed. No CPB was required.
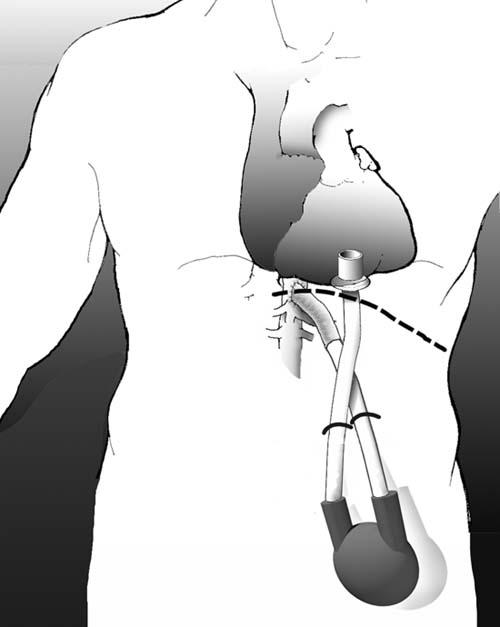
Fig. 1 Implantation of a Thorate® ventricular assist device through a left upper-quadrant abdominal incision.
Results. Over the next 7 days, the patient slowly improved, with normalization of end-organ function. On postoperative day 5, however, the patient, who previously had been neurologically intact, became unresponsive. A computed tomographic scan revealed intracranial hemorrhage. After appropriate consultation with his family, further support was discontinued, and the patient died.
Discussion
Pumps such as the one described here have been placed successfully in thousands of patients with terminal heart failure; however, the mortality rate associated with the surgery remains high. The approach used in this patient may be of value in selected cases, because it avoids the deleterious effects of CPB and the blood loss that accompanies repeated sternotomy in hemodynamically compromised and hypocoagulable patients. Although the outcome was not successful in this patient, the cardiac output was normalized successfully by the pump. The patient's death was related to his advanced cardiovascular disease state; earlier use of this device might improve patient outcomes.
Although suturing the outflow graft to the supraceliac aorta is challenging through this exposure, it can be done successfully. Dedicated retractors and tools, if developed, could facilitate this maneuver greatly.
We have used this approach in several patients for the implantation of an intracorporeal continuous flow LVAD (Jarvik 2000, Jarvik Heart, Inc.; New York, NY) and have found that the incision is well tolerated, heals well, and allows the patient to rapidly return to normal activity. To our knowledge, this report documents the 1st use of this technique for the insertion of an extracorporeal pulsatile ventricular assist device for temporary ventricular support.
Footnotes
Address for reprints: William E. Cohn, MD, Texas Heart Institute, MC 2-114A, P.O. Box 20345, Houston, TX 77225-0345
E-mail: wcohn@heart.thi.tmc.edu


