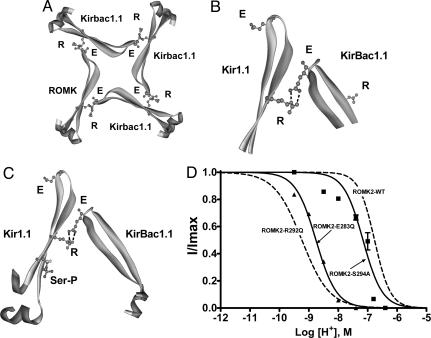
Fig. 5.
Contributions of the E-R salt bridge and phosphorylation to channel activity are independent and additive. (A) Tetrameric ring of Kir channel subunits viewed from above showing the proposed C–C-terminal E-R salt bridge interaction between the glutamate (E283) and the arginine (R292) on an adjacent subunit. Approximately 20 residues on either side of the proposed E-R salt bridge are shown. The model of Kir1.1 was generated by sequence homology to KirBac1.1 and based on the x-ray structure data set of KirBac1.1 (1N7P) using Swiss-Model (http://swissmodel.expasy.org/swiss-model.html). The other three subunits are KirBac1.1. (B) Magnified E-R salt bridge interaction from A. (C) The E-R salt bridge is shown in relation to the modeled phosphorylated serine residue on the Kir1.1 subunit. The serine (S294 in ROMK2) was substituted for a phosphorylated serine; therefore, this figure should be interpreted as a cartoon. Noting this, the cartoon agrees with the experimental data that phosphorylation at the serine is independent of, and does not appear to affect, the E-R salt bridge interaction. (D) pH dose–response relationships for the C-terminal R, E, and S mutants.