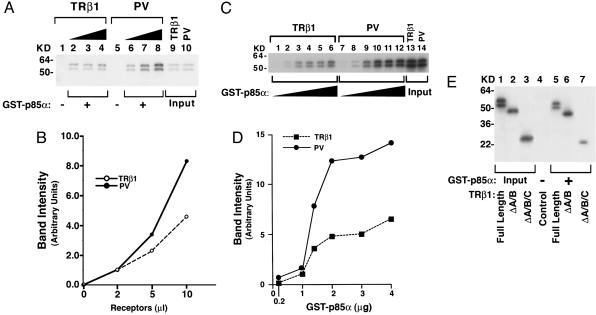
Fig. 2.
p85α protein binds to PV more avidly than to TRβ1.(A) GST–p85α fusion protein was incubated with 2, 5, or 10 μl of 35S-labeled TRβ1 (lanes 2, 3, and 4, respectively) or PV (lanes 6, 7, and 8, respectively) prepared by in vitro translation/transcription. Lanes 1 and 5 were from the incubation of GST with 2 μl of TRβ1 and PV, respectively. Lanes 9 and 10 show the input of in vitro translation/transcription samples (0.2 μl). (B) The band intensity was scanned and quantified by using nih image software. (C) Ten microliters of 35S-labeled TRβ1 (lanes 1–6) or PV (lanes 7–12) prepared by in vitro translation/transcription was incubated with increasing concentrations of bead-bound GST–p85α as described in Materials and Methods. (D) The band intensity was scanned and quantified by using nih image. (E) Identification of the ligand-binding domain of TRβ1 as the interaction site with p85α. Five microliters of 35S-labeled full-length and TRβ1 proteins lacking the A/B domain (ΔA/B) or the A/B/C domains (ΔA/B/C) was synthesized (25) by in vitro transcription/translation and incubated with GST–p85α, as described in Materials and Methods. Lanes 1–3 were the input (0.5 μl of lysates). Lanes are as marked.