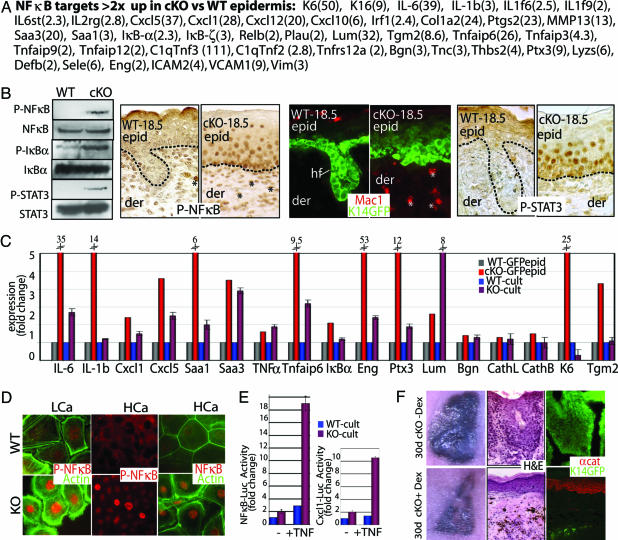
Fig. 3.
Early activation of the NF-κB pathway in α-catenin null epidermis. (A) Putative NF-κB target genes whose mRNAs were scored by microarray as up-regulated in E18.5 cKO vs. WT epidermis. Numbers in parentheses correspond to fold change. (B) NF-κB activation and inflammation in E18.5 cKO skin. (Left) Immunoblot with panel and phospho-specific Abs: P-NF-κB (activated p65 subunit), P-IκBα (inhibited state), and P-STAT3 (activated). (Right) Immunostainings with Abs against P-NF-κB, Mac1 (macrophage-specific), and P-STAT3. Asterisks denote positive dermal nuclei; additional identification of inflammatory cells is in Fig. 9. (C) Real-time PCR of NF-κB target genes. mRNAs were from FACS-purified GFP-positive epidermal cells and cultured keratinocytes (low calcium). Fold change was relative to WT-GFPepid (=1). (D) Anti-NF-κB and P-NF-κB immunofluorescence of KO and WT keratinocytes that are also GFPactin transgenic. Cells were cultured in low-calcium medium (LCa) and high-calcium medium (HCa). (E) NF-κB-luciferase and Cxcl1-luciferase reporter gene assays on cultured keratinocytes (LCa) ± TNF-α. Values shown are relative to Renilla luciferase levels. ∗, t test statistical P ≤ 0.005. (F) Nude mice harboring K14-GFP-expressing E18.5 WT and cKO skin grafts were treated ± Dex to suppress NF-κB activation. Grafts were processed at 30 days for hematoxylin and eosin (H&E) staining, and immunomicroscopy was performed with Ab against α-catenin (αcat). Pigmented melanocytes from grafted skin (C57/Bl6) are black.